September 7, 2022 UPDATE – I’m thrilled to share that the paper with the primary outcomes from the CREATE trial is now published. You can find it on the journal site here, or view an author copy here. You can also see a Twitter thread here, if you are interested in sharing the study with your networks.
Example citation:
Burnside, M; Lewis, D; Crocket, H; et al. Open-Source Automated Insulin Delivery in Type 1 Diabetes. N Engl J Med 2022;387:869-81. DOI:10.1056/NEJMoa2203913
—
(You can also see a previous Twitter thread here summarizing the study results, if you are interested in sharing the study with your networks.)
TLDR: The CREATE Trial was a multi-site, open-labeled, randomized, parallel-group, 24-week superiority trial evaluating the efficacy and safety of an open-source AID system using the OpenAPS algorithm in a modified version of AndroidAPS. Our study found that across children and adults, the percentage of time that the glucose level was in the target range of 3.9-10mmol/L [70-180mg/dL] was 14 percentage points higher among those who used the open-source AID system (95% confidence interval [CI], 9.2 to 18.8; P<0.001) compared to those who used sensor augmented pump therapy; a difference that corresponds to 3 hours 21 minutes more time spent in target range per day. The system did not contribute to any additional hypoglycemia. Glycemic improvements were evident within the first week and were maintained over the 24-week trial. This illustrates that all people with T1D, irrespective of their level of engagement with diabetes self-care and/or previous glycemic outcomes, stand to benefit from AID. This study concluded that open-source AID using the OpenAPS algorithm within a modified version of AndroidAPS, a widely used open-source AID solution, is efficacious and safe.
—
The backstory on this study
We developed the first open source AID in late 2014 and shared it with the world as OpenAPS in February 2015. It went from n=1 to (n=1)*2 and up from there. Over time, there were requests for data to help answer the question “how do you know it works (for anybody else)?”. This led to the first survey in the OpenAPS community (published here), followed by additional retrospective studies such as this one analyzing data donated by the community, prospective studies, and even an in silico study of the algorithm. Thousands of users chose open source AID, first because there was no commercial AID, and later because open source AID such as the OpenAPS algorithm was more advanced or had interoperability features or other benefits such as quality of life improvements that they could not find in commercial AID (or because they were still restricted from being able to access or afford commercial AID options). The pile of evidence kept growing, and each study has shown safety and efficacy matching or surpassing commercial AID systems (such as in this study), yet still, there was always the “but there’s no RCT showing safety!” response.
After Martin de Bock saw me present about OpenAPS and open source AID at ADA Scientific Sessions in 2018, we literally spent an evening at the dinner table drawing the OpenAPS algorithm on a napkin at the table to illustrate how OpenAPS works in fine grained detail (as much as one can do on napkin drawings!) and dreamed up the idea of an RCT in New Zealand to study the open source AID system so many were using. We sought and were granted funding by New Zealand’s Health Research Council, published our protocol, and commenced the study.
—
This is my high level summary of the study and some significant aspects of it.
Study Design:
This study was a 24-week, multi-centre randomized controlled trial in children (7–15 years) and adults (16–70 years) with type 1 diabetes comparing open-source AID (using the OpenAPS algorithm within a version of AndroidAPS implemented in a smartphone with the DANA-i™ insulin pump and Dexcom G6® CGM), to sensor augmented pump therapy. The primary outcome was change in the percent of time in target sensor glucose range (3.9-10mmol/L [70-180mg/dL]) from run-in to the last two weeks of the randomized controlled trial.
- This is a LONG study, designed to look for rare adverse events.
- This study used the OpenAPS algorithm within a modified version of AndroidAPS, meaning the learning objectives were adapted for the purpose of the study. Participants spent at least 72 hours in “predictive low glucose suspend mode” (known as PLGM), which corrects for hypoglycemia but not hyperglycemia, before proceeding to the next stage of closed loop which also then corrected for hyperglycemia.
- The full feature set of OpenAPS and AndroidAPS, including “supermicroboluses” (SMB) were able to be used by participants throughout the study.
Results:
Ninety-seven participants (48 children and 49 adults) were randomized.
Among adults, mean time in range (±SD) at study end was 74.5±11.9% using AID (Δ+ 9.6±11.8% from run-in; P<0.001) with 68% achieving a time in range of >70%.
Among children, mean time in range at study end was 67.5±11.5% (Δ+ 9.9±14.9% from run-in; P<0.001) with 50% achieving a time in range of >70%.
Mean time in range at study end for the control arm was 56.5±14.2% and 52.5±17.5% for adults and children respectively, with no improvement from run-in. No severe hypoglycemic or DKA events occurred in either arm. Two participants (one adult and one child) withdrew from AID due to frustrations with hardware issues.
- The pump used in the study initially had an issue with the battery, and there were lots of pumps that needed refurbishment at the start of the study.
- Aside from these pump issues, and standard pump site/cannula issues throughout the study (that are not unique to AID), there were no adverse events reported related to the algorithm or automated insulin delivery.
- Only two participants withdrew from AID, due to frustration with pump hardware.
- No severe hypoglycemia or DKA events occurred in either study arm!
- In fact, use of open source AID improved time in range without causing additional hypoglycemia, which has long been a concern of critics of open source (and all types of) AID.
- Time spent in ‘level 1’ and ‘level 2’ hyperglycemia was significantly lower in the AID group as well compared to the control group.
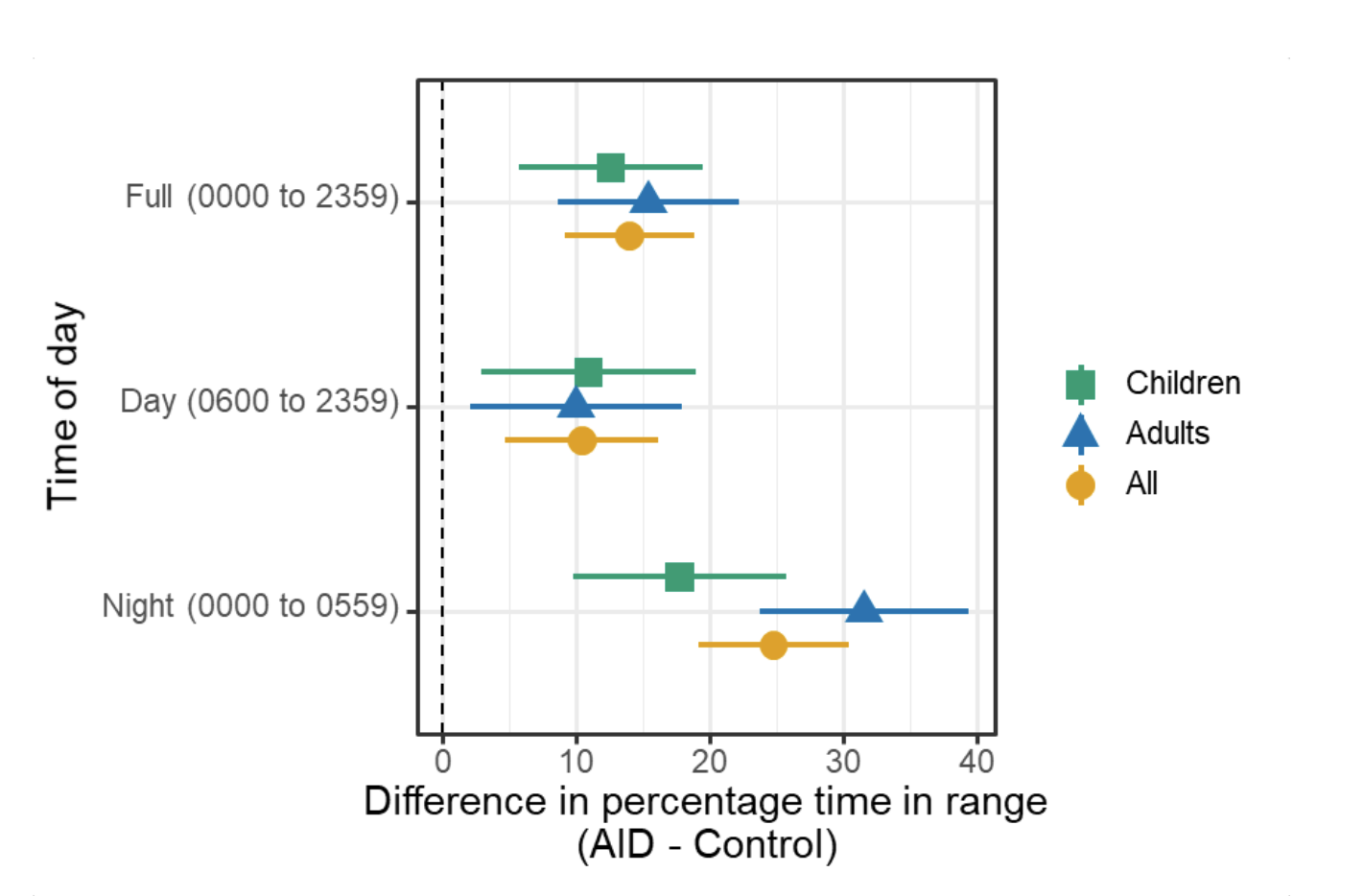
In the primary analysis, the mean (±SD) percentage of time that the glucose level was in the target range (3.9 – 10mmol/L [70-180mg/dL]) increased from 61.2±12.3% during run-in to 71.2±12.1% during the final 2-weeks of the trial in the AID group and decreased from 57.7±14.3% to 54±16% in the control group, with a mean adjusted difference (AID minus control at end of study) of 14.0 percentage points (95% confidence interval [CI], 9.2 to 18.8; P<0.001). No age interaction was detected, which suggests that adults and children benefited from AID similarly.
- The CREATE study found that across children and adults, the percentage of time that the glucose level was in the target range of 3.9-10mmol/L [70-180mg/dL] was 14.0 percentage points higher among those who used the open-source AID system compared to those who used sensor augmented pump therapy.
- This difference reflects 3 hours 21 minutes more time spent in target range per day!
- For children AID users, they spent 3 hours 1 minute more time in target range daily (95% CI, 1h 22m to 4h 41m).
- For adult AID users, they spent 3 hours 41 minutes more time in target range daily (95% CI, 2h 4m to 5h 18m).
- Glycemic improvements were evident within the first week and were maintained over the 24-week trial. Meaning: things got better quickly and stayed so through the entire 24-week time period of the trial!
- AID was most effective at night.
One thing I think is worth making note of is that one criticism of previous studies with open source AID is regarding the self-selection effect. There is the theory that people do better with open source AID because of self-selection and self-motivation. However, the CREATE study recruited a diverse cohort of participants, and the study findings (as described above) match all previous reports of safety and efficacy outcomes from previous studies. The CREATE study also found that the greatest improvements in TIR were seen in participants with lowest TIR at baseline. This means one major finding of the CREATE study is that all people with T1D, irrespective of their level of engagement with diabetes self-care and/or previous glycemic outcomes, stand to benefit from AID.
This therefore means there should be NO gatekeeping by healthcare providers or the healthcare system to restrict AID technology from people with insulin-requiring diabetes, regardless of their outcomes or experiences with previous diabetes treatment modalities.
There is also no age effect observed in the trail, meaning that the results of the CREATE Trial demonstrated that open-source AID is safe and effective in children and adults with type 1 diabetes. If someone wants to use open source AID, they would likely benefit, regardless of age or past diabetes experiences. If they don’t want to use open source AID or commercial AID…they don’t have to! But the choice should 100% be theirs.
In summary:
- The CREATE trial was the first RCT to look at open source AID, after years of interest in such a study to complement the dozens of other studies evaluating open source AID.
- The conclusion of the CREATE trial is that open-source AID using the OpenAPS algorithm within a version of AndroidAPS, a widely used open-source AID solution, appears safe and effective.
- The CREATE trial found that across children and adults, the percentage of time that the glucose level was in the target range of 3.9-10mmol/L [70-180mg/dL] was 14.0 percentage points higher among those who used the open-source AID system compared to those who used sensor augmented pump therapy; a difference that reflects 3 hours 21 minutes more time spent in target range per day.
- The study recruited a diverse cohort, yet still produced glycemic outcomes consistent with existing open-source AID literature, and that compare favorably to commercially available AID systems. Therefore, the CREATE Trial indicates that a range of people with type 1 diabetes might benefit from open-source AID solutions.
—
Huge thanks to each and every participant and their families for their contributions to this study! And ditto, big thanks to the amazing, multidisciplinary CREATE study team for their work on this study.
—
September 7, 2022 UPDATE – I’m thrilled to share that the paper with the primary outcomes from the CREATE trial is now published. You can find it on the journal site here, or like all of the research I contribute to, access an author copy on my research paper.
Example citation:
Burnside, M; Lewis, D; Crocket, H; et al. Open-Source Automated Insulin Delivery in Type 1 Diabetes. N Engl J Med 2022;387:869-81. DOI:10.1056/NE/Moa2203913
—
Note that the continuation phase study results are slated to be presented this fall at another conference!




 I started with a basic sketch of an idea to run it by Scott and a few other people to test the idea. I’m not much for drawing, so it was a *very* rough sketch. But the analogy seemed to resonate, so I moved on to mocking up a basic version on the computer. (I went down a rabbit hole because I thought it would be neat to make an animated video for people to see and share online, to accompany the book. But I don’t know how to illustrate on the computer, let alone animate, so I tried an open source illustration program called Synfig, then several other illustrator programs that were open source to do the basic design to import into Synfig to animate, but then realized what I had in mind was so simple that basic transitions and animations in PowerPoint would suffice for my animated video.) PowerPoint is also
I started with a basic sketch of an idea to run it by Scott and a few other people to test the idea. I’m not much for drawing, so it was a *very* rough sketch. But the analogy seemed to resonate, so I moved on to mocking up a basic version on the computer. (I went down a rabbit hole because I thought it would be neat to make an animated video for people to see and share online, to accompany the book. But I don’t know how to illustrate on the computer, let alone animate, so I tried an open source illustration program called Synfig, then several other illustrator programs that were open source to do the basic design to import into Synfig to animate, but then realized what I had in mind was so simple that basic transitions and animations in PowerPoint would suffice for my animated video.) PowerPoint is also 




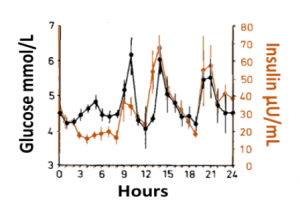 Blood glucose and insulin exhibit coupled biological rhythms at multiple timescales, including hours (ultradian, UR) and the day (circadian, CR) in individuals without diabetes. The presence and stability of these rhythms are associated with healthy glucose control in individuals without diabetes. (See right, adapted from
Blood glucose and insulin exhibit coupled biological rhythms at multiple timescales, including hours (ultradian, UR) and the day (circadian, CR) in individuals without diabetes. The presence and stability of these rhythms are associated with healthy glucose control in individuals without diabetes. (See right, adapted from 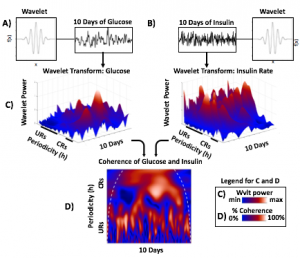
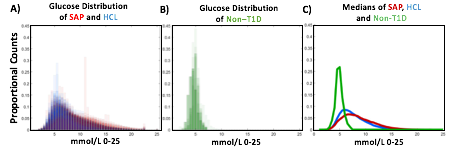
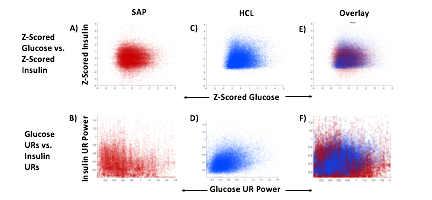
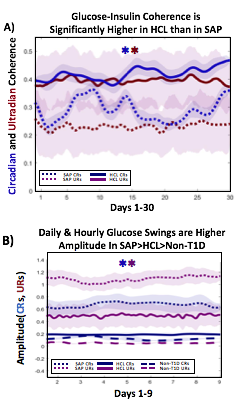 A) Circadian (blue) and 3-6 hour ultradian (maroon) coherence of glucose and insulin in HCL (solid) and SAP (dotted) users. Transparent shading indicates standard deviation. Although both HCL and SAP individuals have lower coherence than would be expected in a non-T1D individual, HCL CR and UR coherence are significantly greater than SAP CR and UR coherence (paired t-test p= 1.51*10-7 t=-5.77 and p= 5.01*10-14 t=-9.19, respectively). This brings HCL users’ glucose and insulin closer to the canonical non-T1D phenotype than SAP users’.
A) Circadian (blue) and 3-6 hour ultradian (maroon) coherence of glucose and insulin in HCL (solid) and SAP (dotted) users. Transparent shading indicates standard deviation. Although both HCL and SAP individuals have lower coherence than would be expected in a non-T1D individual, HCL CR and UR coherence are significantly greater than SAP CR and UR coherence (paired t-test p= 1.51*10-7 t=-5.77 and p= 5.01*10-14 t=-9.19, respectively). This brings HCL users’ glucose and insulin closer to the canonical non-T1D phenotype than SAP users’.

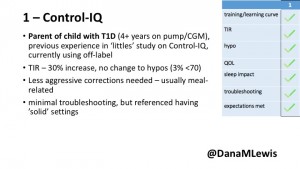
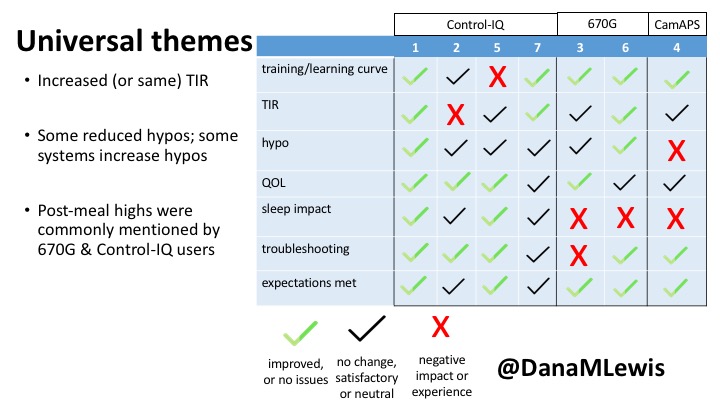
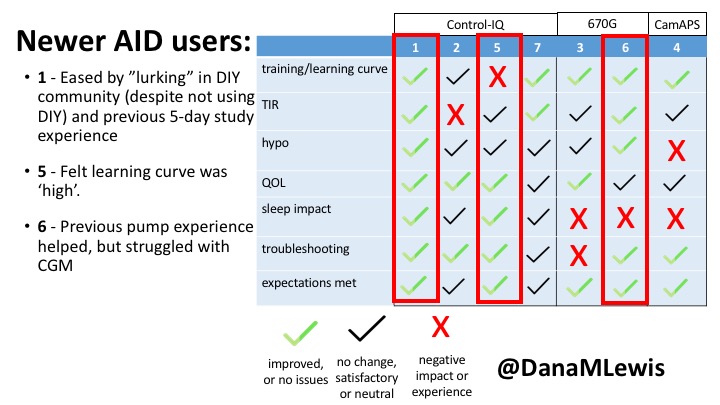
 1 – A parent of a child using Control-IQ (off-label), with 30% increase in TIR with no increased hypoglycemia. They spend less time correcting than before; less time thinking about diabetes; and “get solid uninterrupted sleep for the first time since diagnosis”. They wish they had remote bolusing, more system information available in remote monitoring on phones. They miss using the system during the 2 hour CGM warmup, and found the system dealt well with growth spurt hormones but not as well with underestimated meals.
1 – A parent of a child using Control-IQ (off-label), with 30% increase in TIR with no increased hypoglycemia. They spend less time correcting than before; less time thinking about diabetes; and “get solid uninterrupted sleep for the first time since diagnosis”. They wish they had remote bolusing, more system information available in remote monitoring on phones. They miss using the system during the 2 hour CGM warmup, and found the system dealt well with growth spurt hormones but not as well with underestimated meals.
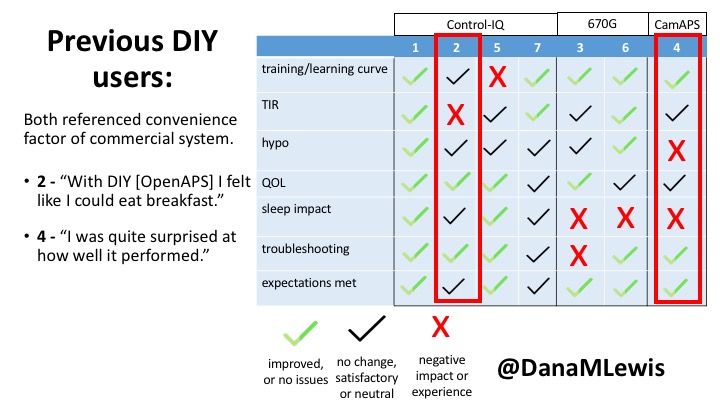
 2 – An adult male with T1D who previously used DIYAPS saw 5-10% decrease in TIR (but it’s on par with other participants’ TIR) with Control-IQ, and is very pleased by the all-in-one convenience of his commercial system.He misses autosensitivity (a short-term learning feature of how insulin needs may very from base settings) from DIYAPS and has stopped eating breakfast, since he found it couldn’t manage that well. He is doing more manual corrections than he was before.
2 – An adult male with T1D who previously used DIYAPS saw 5-10% decrease in TIR (but it’s on par with other participants’ TIR) with Control-IQ, and is very pleased by the all-in-one convenience of his commercial system.He misses autosensitivity (a short-term learning feature of how insulin needs may very from base settings) from DIYAPS and has stopped eating breakfast, since he found it couldn’t manage that well. He is doing more manual corrections than he was before.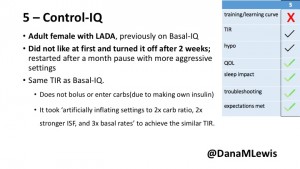
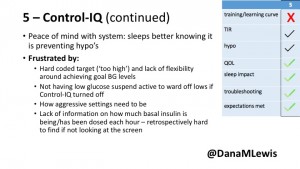 5 – An adult female with LADA started, stopped, and started using Control-IQ, getting the same TIR that she had before on Basal-IQ. It took artificially inflating settings to achieve these similar results. She likes peace of mind to sleep while the system prevents hypoglycemia. She is frustrated by ‘too high’ target; not having low prevention if she disables Control-IQ; and how much she had to inflate settings to achieve her outcomes. It’s hard to know how much insulin the system gives each hour (she still produces some of own insulin).
5 – An adult female with LADA started, stopped, and started using Control-IQ, getting the same TIR that she had before on Basal-IQ. It took artificially inflating settings to achieve these similar results. She likes peace of mind to sleep while the system prevents hypoglycemia. She is frustrated by ‘too high’ target; not having low prevention if she disables Control-IQ; and how much she had to inflate settings to achieve her outcomes. It’s hard to know how much insulin the system gives each hour (she still produces some of own insulin).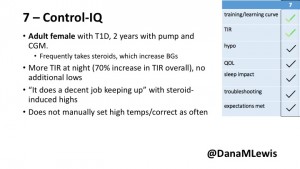
 7 – An adult female with T1D who frequently has to take steroids for other reasons, causing increased BGs. With Control-IQ, she sees 70% increase in TIR overall and increased TIR overnight, and found it does a ‘decent job keeping up’ with steroid-induced highs. She also wants to run ‘tighter’ and have an adjustable target, and does not ever run in sleep mode so that she can always get the bolus corrections that are more likely to bring her closer to target.
7 – An adult female with T1D who frequently has to take steroids for other reasons, causing increased BGs. With Control-IQ, she sees 70% increase in TIR overall and increased TIR overnight, and found it does a ‘decent job keeping up’ with steroid-induced highs. She also wants to run ‘tighter’ and have an adjustable target, and does not ever run in sleep mode so that she can always get the bolus corrections that are more likely to bring her closer to target.
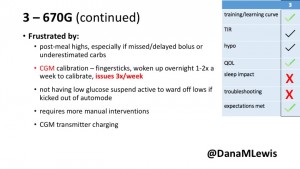 3 – An adult male with T1D using 670G for 3 years didn’t observe any changes to A1c or TIR, but is pleased with his outcomes, especially with the ability to handle his activity levels by using the higher activity target. He is frustrated by the CGM and is woken up 1-2x a week to calibrate overnight. He wishes he could still have low glucose suspend even if he’s kicked out of automode due to calibration issues. He also commented on post-meal highs and more manual interventions.
3 – An adult male with T1D using 670G for 3 years didn’t observe any changes to A1c or TIR, but is pleased with his outcomes, especially with the ability to handle his activity levels by using the higher activity target. He is frustrated by the CGM and is woken up 1-2x a week to calibrate overnight. He wishes he could still have low glucose suspend even if he’s kicked out of automode due to calibration issues. He also commented on post-meal highs and more manual interventions.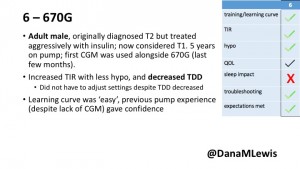
 6 – Another adult male user with 670G was originally diagnosed with T2 (now considered T1) with a very high total daily insulin use that was able to decrease significantly when switching to AID. He’s happy with increased TIR and less hypo, plus decreased TDD. Due to #COVID19, he did virtually training but would have preferred in-person. He has 4-5 alerts/day and is woken up every other night due to BG alarms or calibration. He does not like the time it takes to charge CGM transmitter, in addition to sensor warmup.
6 – Another adult male user with 670G was originally diagnosed with T2 (now considered T1) with a very high total daily insulin use that was able to decrease significantly when switching to AID. He’s happy with increased TIR and less hypo, plus decreased TDD. Due to #COVID19, he did virtually training but would have preferred in-person. He has 4-5 alerts/day and is woken up every other night due to BG alarms or calibration. He does not like the time it takes to charge CGM transmitter, in addition to sensor warmup.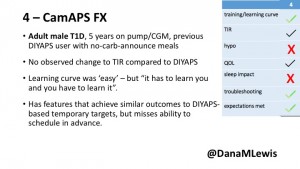
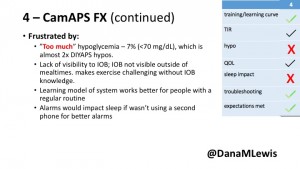 4 – The last participant is an adult male with T1 who previously used DIYAPS but was able to test-drive the CamAPS FX. He saw no TIR change to DIYAPS (which pleased him) and thought the learning curve was easy – but he had to learn the system and let it learn him. He experienced ‘too much’ hypoglycemia (~7% <70mg/dL, 2x his previous), and found it challenging to not have visibility of IOB. He also found the in-app CGM alarms annoying. He noted the system may work better for people with regular routines.
4 – The last participant is an adult male with T1 who previously used DIYAPS but was able to test-drive the CamAPS FX. He saw no TIR change to DIYAPS (which pleased him) and thought the learning curve was easy – but he had to learn the system and let it learn him. He experienced ‘too much’ hypoglycemia (~7% <70mg/dL, 2x his previous), and found it challenging to not have visibility of IOB. He also found the in-app CGM alarms annoying. He noted the system may work better for people with regular routines.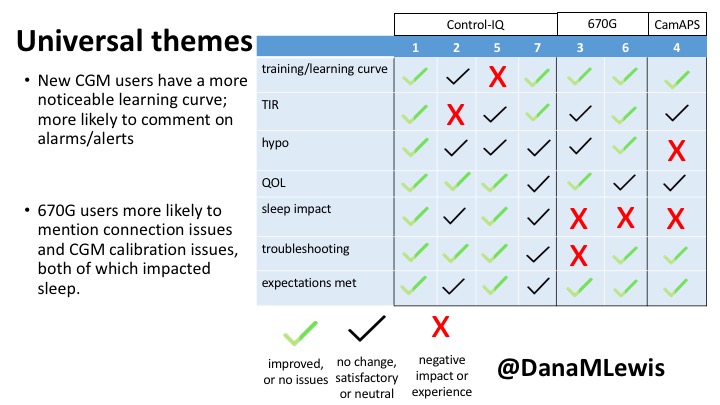



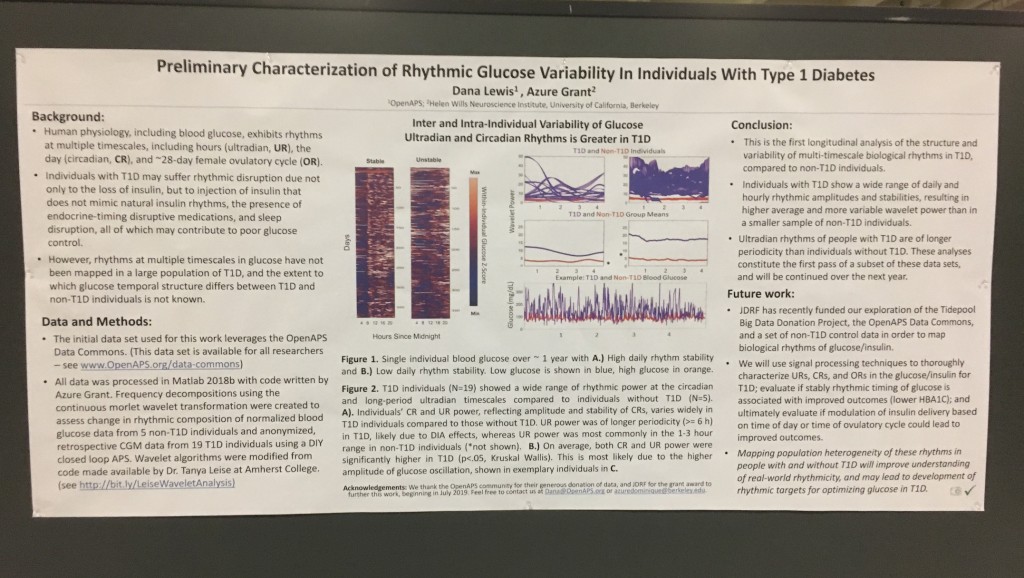
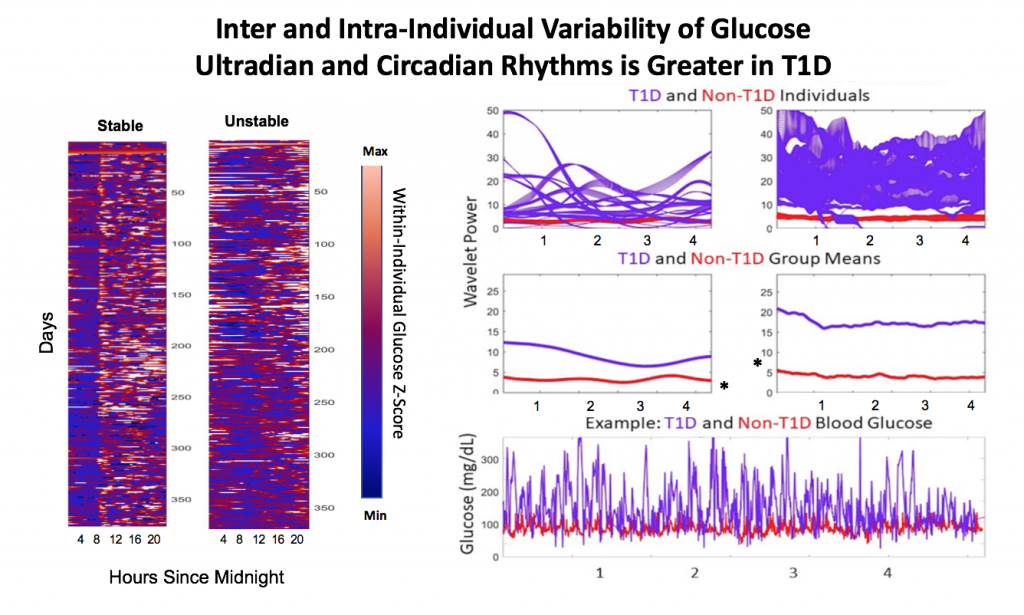

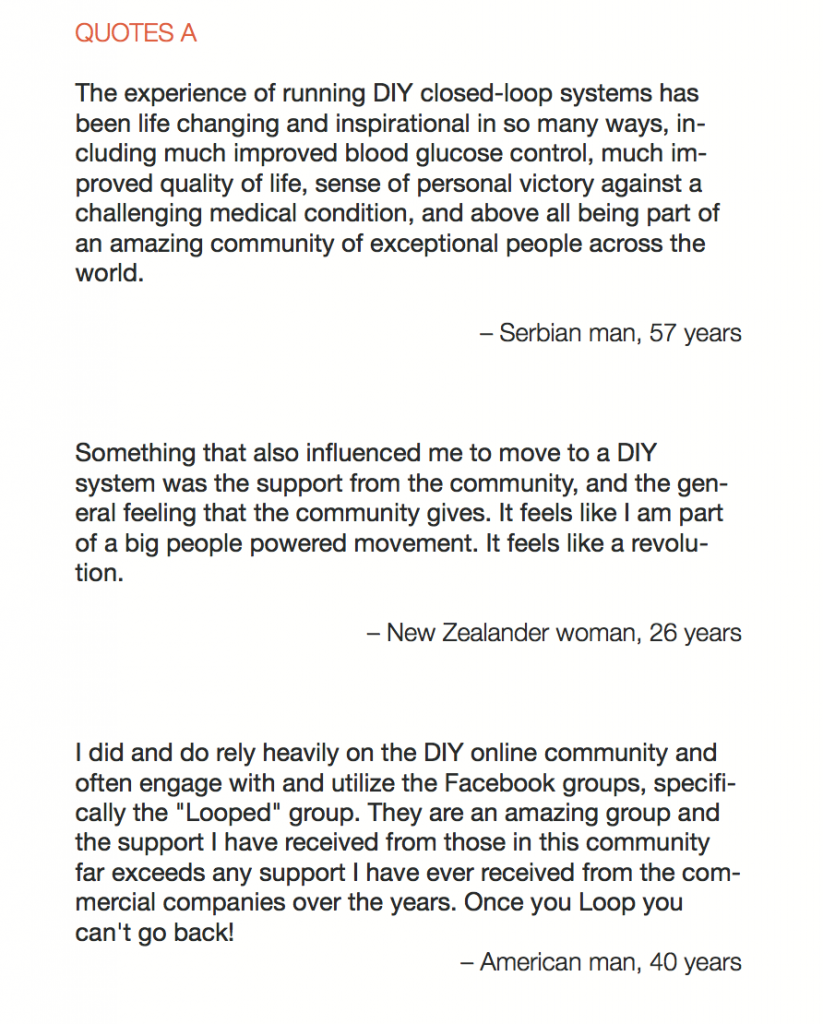



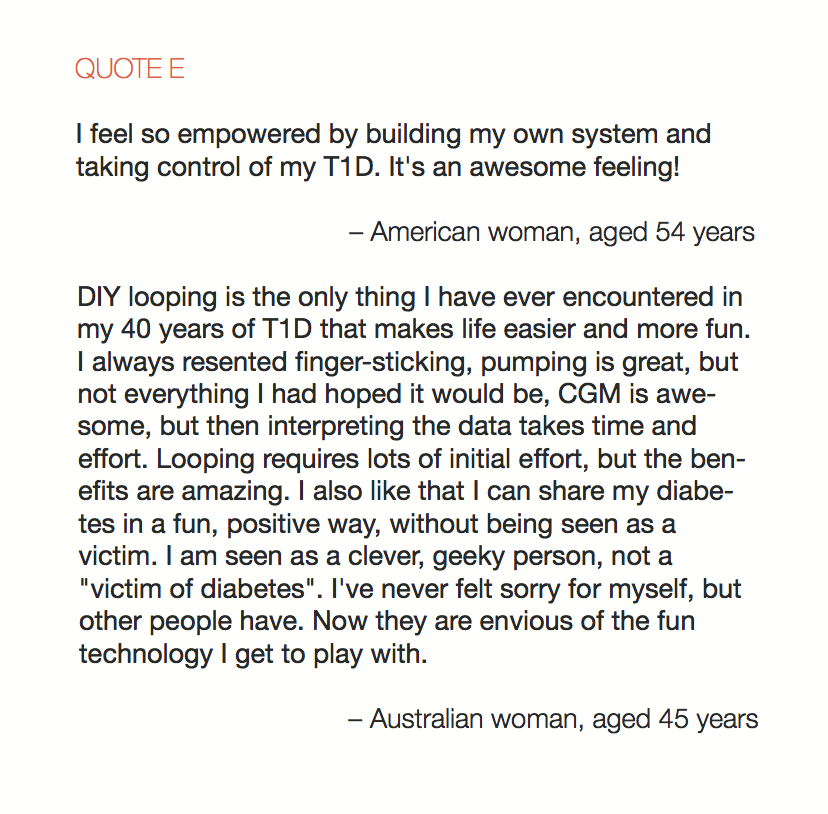

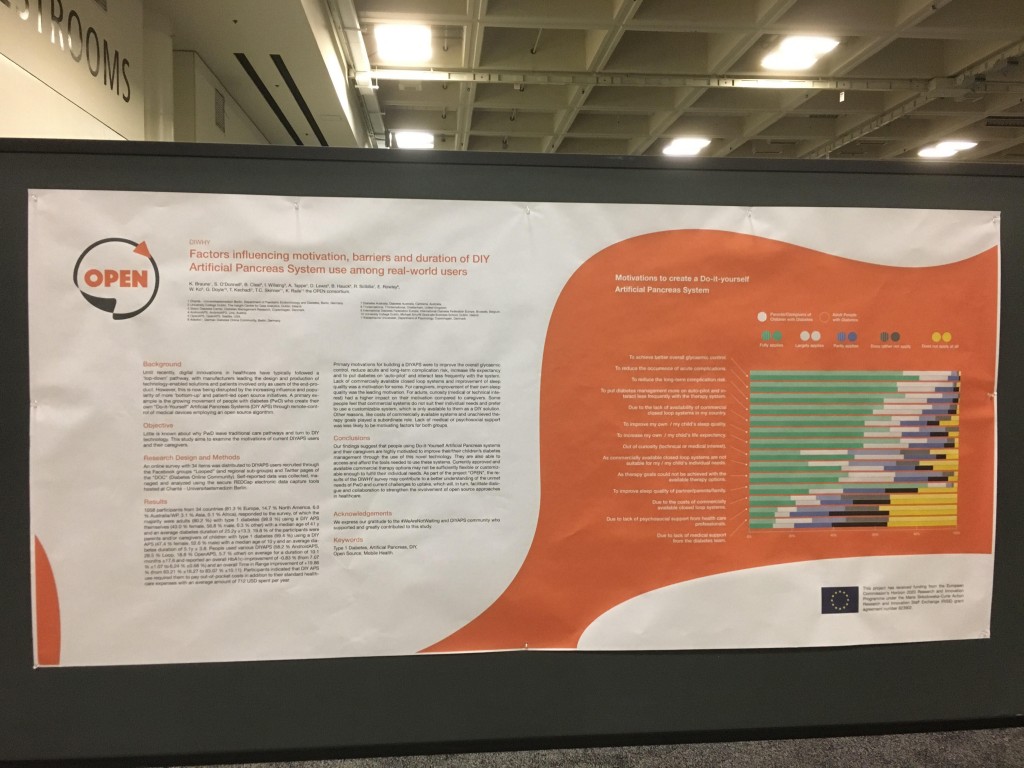
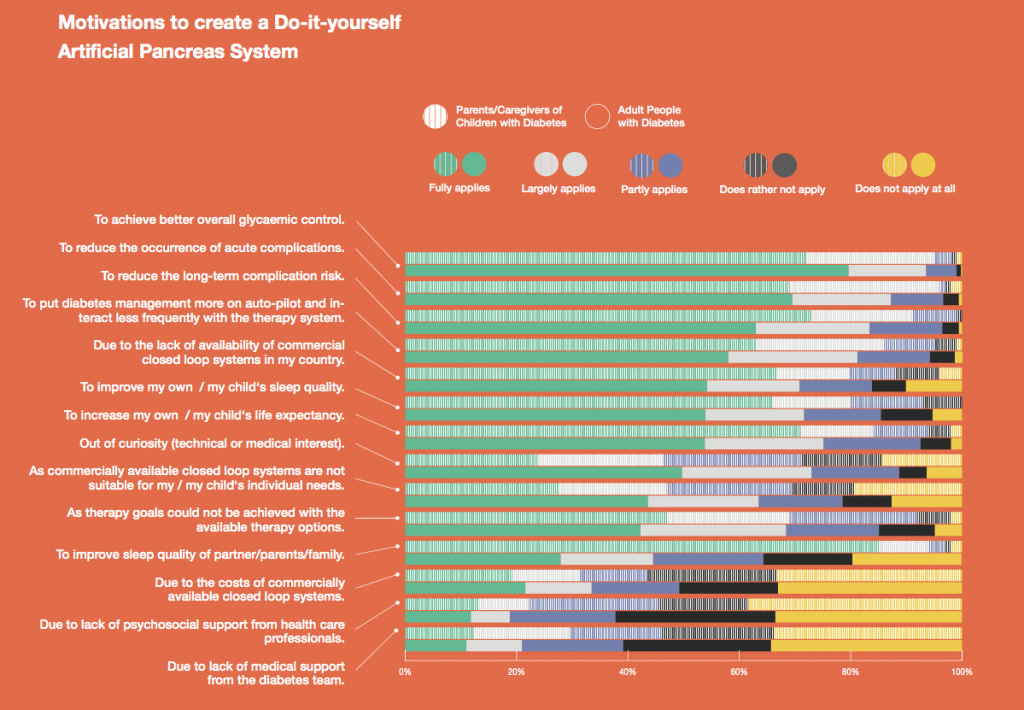
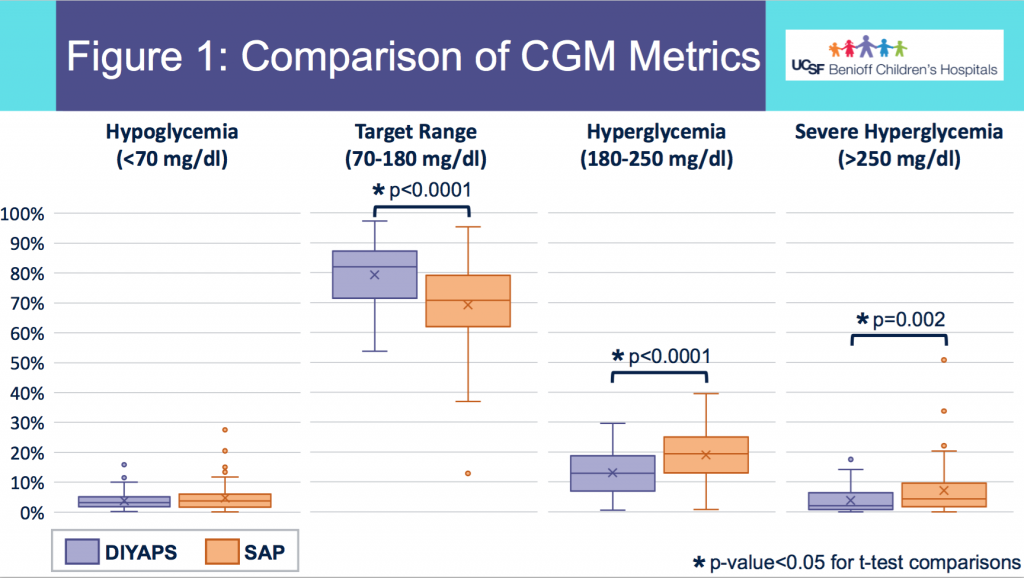
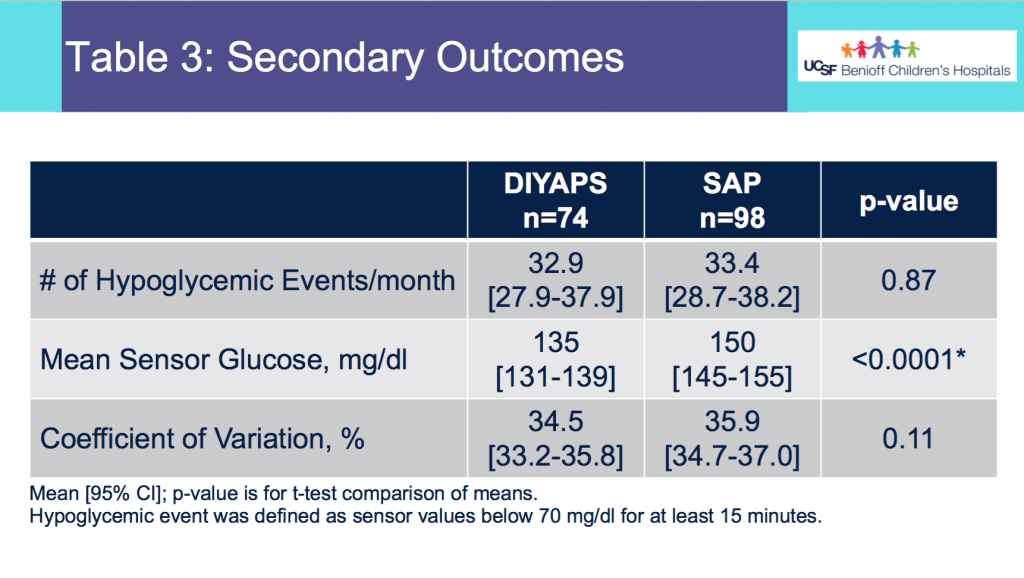
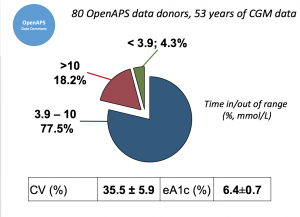 Looking at results for #OpenAPS data donors post-looping initiation, CV was 35.5±5.9, while eA1c was 6.4±0.7. TIR (3.9-10mmol/L) was 77.5%. Time spent >10 was 18.2%; time <3.9 was 4.3%.
Looking at results for #OpenAPS data donors post-looping initiation, CV was 35.5±5.9, while eA1c was 6.4±0.7. TIR (3.9-10mmol/L) was 77.5%. Time spent >10 was 18.2%; time <3.9 was 4.3%.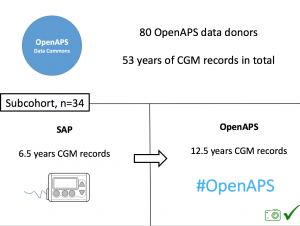 We selected a subcohort of n=34 who had data available from before DIY closed looping initiation (6.5 years combined of CGM records), as well as data from after (12.5 years of CGM records).
We selected a subcohort of n=34 who had data available from before DIY closed looping initiation (6.5 years combined of CGM records), as well as data from after (12.5 years of CGM records).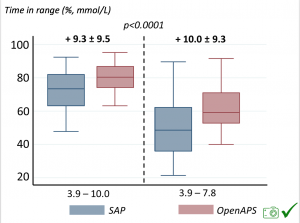 Time in a range significantly increased for both wider (3.9-10 mmol/L) and tighter (3.9-7.8 mmol/L) ranges.
Time in a range significantly increased for both wider (3.9-10 mmol/L) and tighter (3.9-7.8 mmol/L) ranges.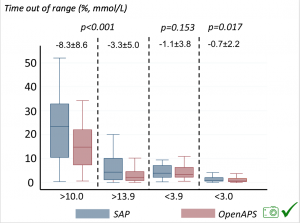 Time spent out of range decreased. % time spent >10 mmol/L decreased -8.3±8.6 (p<0.001); >13 mmol/L decreased -3.3±5.0 (p<0.001). Change in % time spent <3.9 mmol/L (-1.1±3.8 (p=0.153)), and <3.0 mmol/L (-0.7±2.2 (p=0.017)) was not significant.
Time spent out of range decreased. % time spent >10 mmol/L decreased -8.3±8.6 (p<0.001); >13 mmol/L decreased -3.3±5.0 (p<0.001). Change in % time spent <3.9 mmol/L (-1.1±3.8 (p=0.153)), and <3.0 mmol/L (-0.7±2.2 (p=0.017)) was not significant.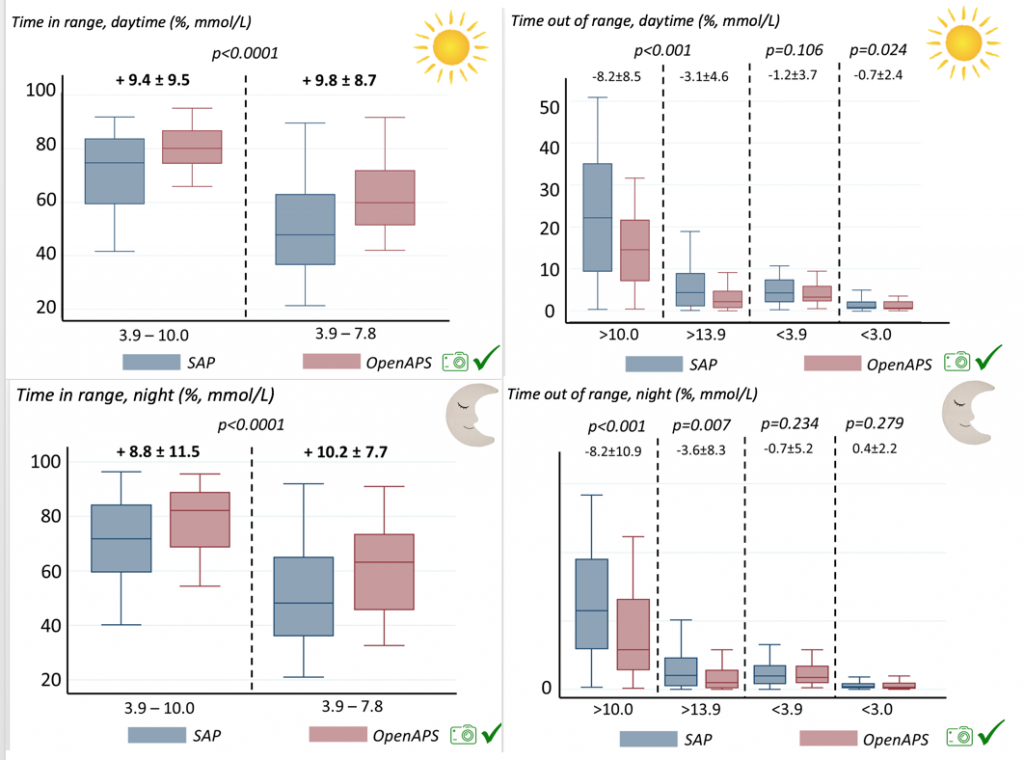
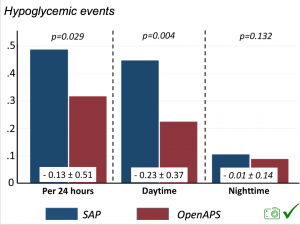 There were less CGM records in the hypoglycemic range after initiating DIYAPS.
There were less CGM records in the hypoglycemic range after initiating DIYAPS.


Recent Comments