It’s been two years since my first DIY closed loop and almost two years since OpenAPS (the vision and resulting ecosystem to help make artificial pancreas technology, DIY or otherwise, more quickly available to more people living with diabetes) was created. I’ve spent time here (on DIYPS.org) talking about a variety of things that are applicable to people who are DIY closed looping, but also focusing on things (like how to “soak” a CGM sensorr and how to do “eating soon” mode) that may be (in my opinion) universally applicable.
However, I think it’s worth recapping some of the amazing work that’s been done in the OpenAPS ecosystem over the past year, sometimes behind the scenes, because there are some key features and tools that have been added in that seem small, but are really impactful for people living with DIY closed loops.
- Advanced meal assist (aka AMA)
- This is an “advanced feature” that can be turned on by OpenAPS users, and, with reliable entry of carb information, will help the closed loop assist sooner with a post-meal BG rise where there is mis-timed or insufficient insulin coverage for the meal. It’s easy to use, because the PWD only has to put carbs and a bolus in – then AMA acts based on the observed absorption. This means that if absorption is delayed because you walk home from dinner, have gastroparesis, etc., it backs off and wait until the carbs actually start taking effect (even if it is later than the human would expect).
- We also now have the purple line predictions back in Nightscout to visualize some of these predictions. This is a hallmark of the original iob-cob branch in Nightscout that Scott and I originally created, that took my COB calculated by DIYPS and visualized the resulting BG graph. With AMA, there are actually 3 purple lines displayed when there is carb activity. As described here in the OpenAPS docs, the top purple line assumes 10 mg/dL/5m carb (0.6 mmol/L/5m) absorption and is most accurate right after eating before carb absorption ramps up. The line that is usually in the middle is based on current carb absorption trends and is generally the most accurate once carb absorption begins; and the bottom line assumes no carb absorption and reflects insulin only. Having the 3 lines is helpful for when you do something out of the ordinary following a meal (taking a walk; taking a shower; etc.) and helps a human decide if they need to do anything or if the loop will be able to handle the resulting impact of those decisions.
- The approach with a “preferences” file
- This is the file where people can adjust default safety and other parameters, like maxIOB which defaults to 0 during a standard setup, ultimately creating a low-glucose-suspend-mode closed loop when people are first setting up their closed loops. People have to intentionally change this setting to allow the system to high temp above a netIOB = 0 amount, which is an intended safety-first approach.
- One particular feature (“override_high_target_with_low”) makes it easier for secondary caregivers (like school nurses) to do conservative boluses at lunch/snack time, and allow the closed loop to pick up from there. The secondary caregiver can use the bolus wizard, which will correct down to the high end of the target; and setting this value in preferences to “true” allows the closed loop to target the low end of the target. Based on anecdotal reports from those using it, this feature sounds like it’s prevented a lot of (unintentional, diabetes is hard) overreacting by secondary caregivers when the closed loop can more easily deal with BG fluctuations. The same for “carbratio_adjustmentratio”, if parents would prefer for secondary caregivers to bolus with a more conservative carb ratio, this can be set so the closed loop ultimately uses the correct carb amount for any needed additional calculations.
- Autosensitivity
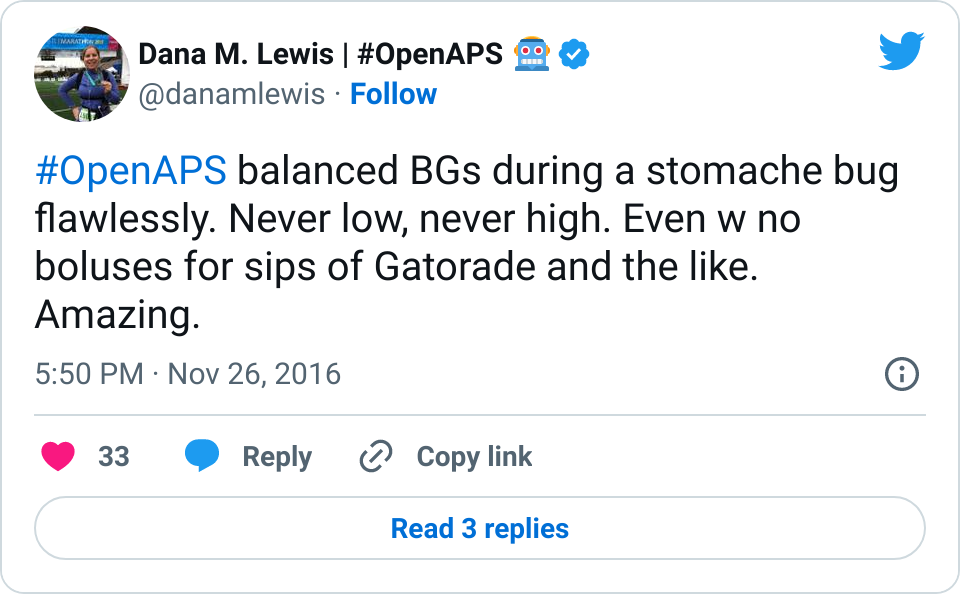
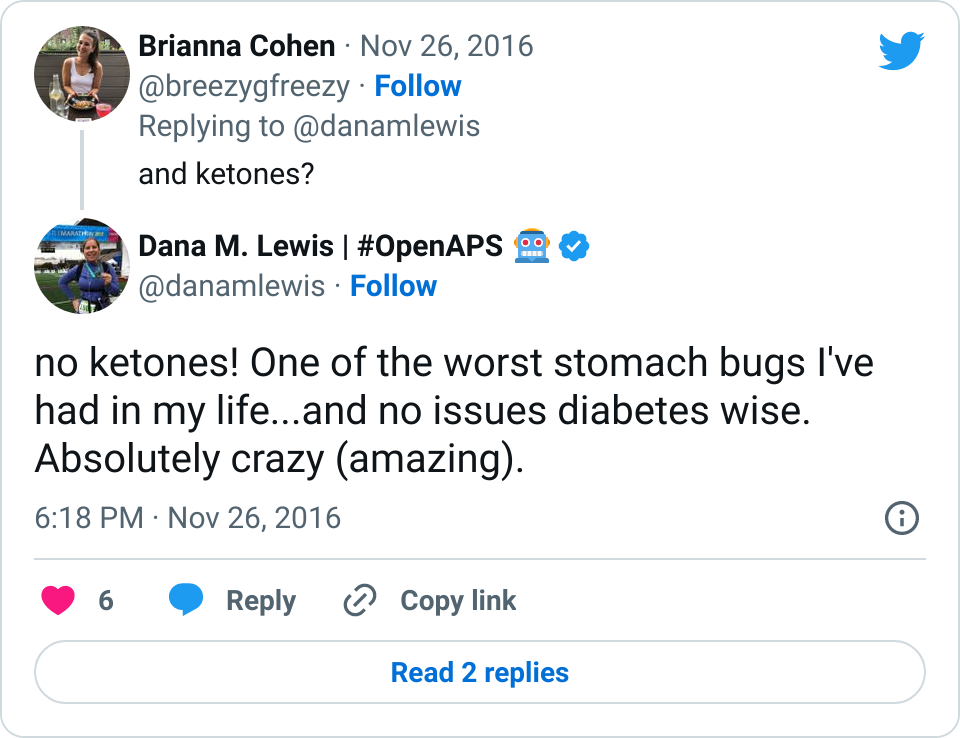
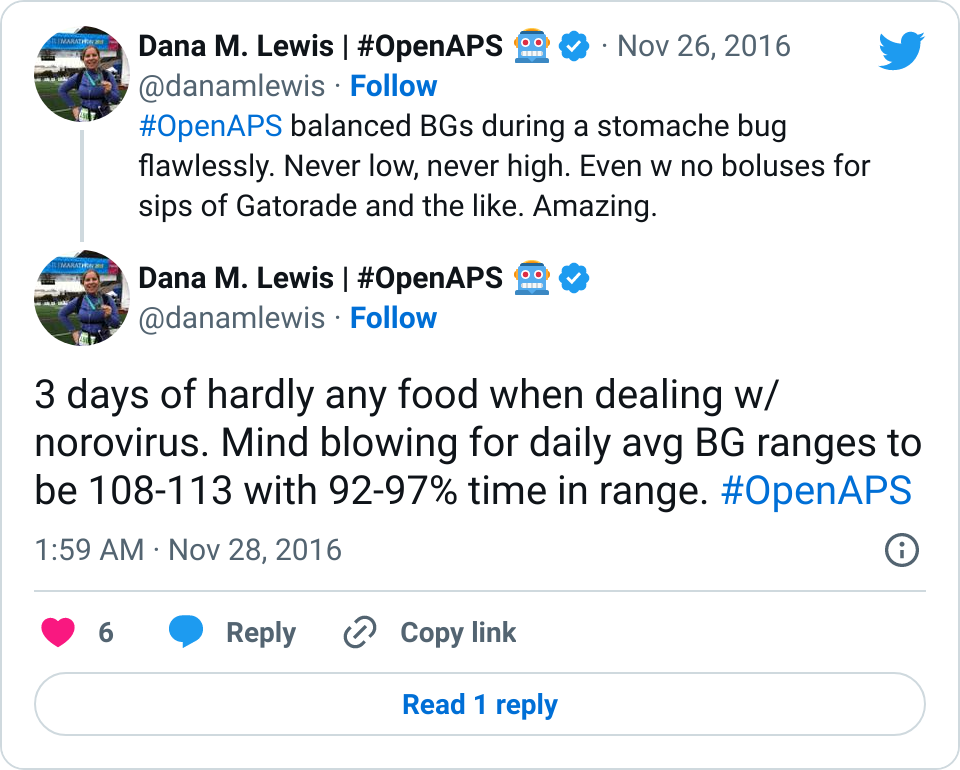
- I’ve written about autosensitivity before and how impressive it has been in the face of a norovirus and not eating to have the closed loop detect excessive sensitivity and be able to deal with it – resulting in 0 lows. It’s also helpful during other minor instances of sensitivity after a few active days; or resistance due to hormone cycles and/or an aging pump site.
- Autosens is a feature that has to be turned on specifically (like AMA) in order for people to utilize it, because it’s making adjustments to ISF and targets and looping accordingly from those values. It also have safety caps that are set and automatically included to limit the amount of adjustment in either direction that autosens can make to any of the parameters.
- Tiny rigs
- Thanks to Intel, we were introduced to a board designer who collaborated with the OpenAPS community and inspired the creation of the “Explorer Board”. It’s a multipurpose board that can be used for home automation and all kinds of things, and it’s another tool in the toolbox of off-the-shelf and commercial hardware that can be used in an OpenAPS setup. It’s enabled us, due to the built in radio stick, to be able to drastically reduce the size of an OpenAPS setup to about the size of two Chapsticks.
- Setup scripts
- As soon as we were working on the Explorer Board, I envisioned that it would be a game changer for increasing access for those who thought a Pi was too big/too burdensome for regular use with a DIY closed loop system. I knew we had a lot of work to do to continue to improve the setup process to cut down on the friction of the setup process – but balancing that with the fact that the DIY part of setting up a closed loop system was and still is incredibly important. We then worked to create the oref0-setup script to streamline the setup process. For anyone building a loop, you still have to set up your hardware and build a system, expressing intention in many places of what you want to do and how…but it’s cut down on a lot of friction and increased the amount of energy people have left, which can instead be focused on reading the code and understanding the underlying algorithm(s) and features that they are considering using.
- Streamlined documentation
- The OpenAPS “docs” are an incredible labor of love and a testament to dozens and dozens of people who have contributed by sharing their knowledge about hardware, software, and the process it takes to weave all of these tools together. It has gotten to be very long, but given the advent of the Explorer Board hardware and the setup scripts, we were able to drastically streamline the docs and make it a lot easier to go from phase 0 (get and setup hardware, depending on the kind of gear you have); to phase 1 (monitoring and visualizing tools, like Nightscout); to phase 2 (actually setup openaps tools and build your system); to phase 3 (starting with a low glucose suspend only system and how to tune targets and settings safely); to phase 4 (iterating and improving on your system with advanced features, if one so desires). The “old” documentation and manual tool descriptions are still in the docs, but 95% of people don’t need them.
- IFTTT and other tool integrations
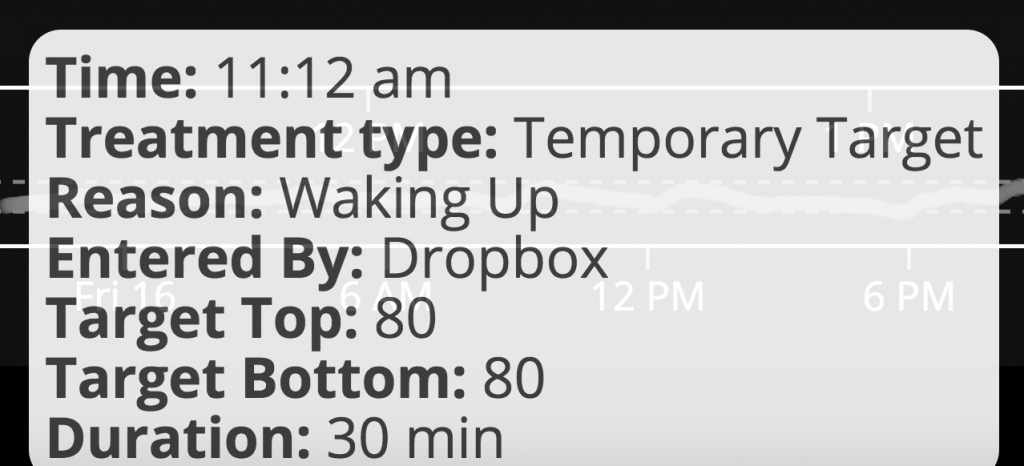
- It’s definitely worth calling out the integration with IFTTT that allows people to use things like Alexa, Siri, Pebble watches, Google Assistant (and just about anything else you can think of), to easily enter carbs or “modes” for OpenAPS to use, or to easily get information about the status of the system. (My personal favorite piece of this is my recent “hack” to automatically have OpenAPS trigger a “waking up” mode to combat hormone-driven BG increases that happen when I start moving around in the morning – but without having to remember to set the mode manually!)
..and that was all just things the community has done in 2016!  There are some other exciting things that are in development and being tested right now by the community, and I look forward to sharing more as this advanced algorithm development continues.
There are some other exciting things that are in development and being tested right now by the community, and I look forward to sharing more as this advanced algorithm development continues.
Happy New Year, everyone!






Recent Comments