You probably heard that a commercial hybrid closed loop (the 670G) has been approved by the U.S. FDA and, like everyone else, are wondering what that means for #OpenAPS.
First, here’s our initial reaction:
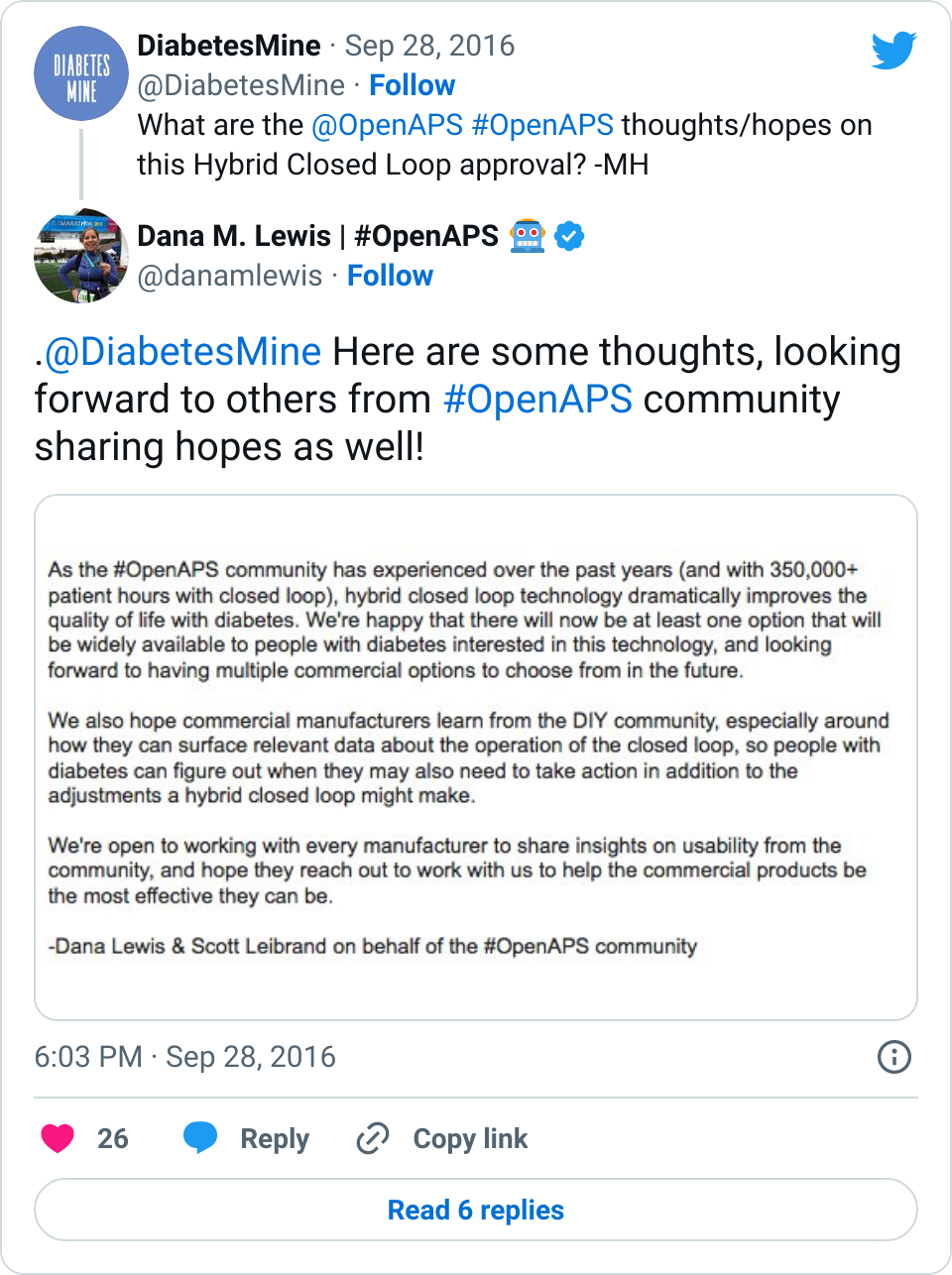
And here are some longer form thoughts:
- Yes, this is exciting. FDA moved months more quickly then expected (hmm, we are sensing a theme when the #WeAreNotWaiting community is involved ;)) to get this tech approved. And as we’ve experienced (check out this self-reported outcomes study with better outcomes than the pivotal trial for this new device), the results of using a hybrid closed loop are outstanding. It’s disappointing that they won’t be ready to ship until Spring 2017, but…
- …This means the company has time to work on user guides and usability. As we’ve told every device company we’ve encountered, we (the #OpenAPS community) are happy to share everything we’ve learned. And we have learned a lot, including what it takes to trust a system, how much info is needed to help determine if additional human action is needed, what to do in all kinds of real-world situations, and more. We hope the companies continue to work with people with diabetes who have experience with this technology from both clinical trials and the DIY world, where we’ve racked up 350,000+ hours with this type of technology. Because setting expectations with users for this technology will be key for successful and sustained adoption.
This doesn’t really mean anything for #OpenAPS, though. The first generation of AP technology is similar to #OpenAPS in that it’s a hybrid closed loop that still requires the human to input carbs into the system, but it unfortunately has a set point that can not be adjusted below 120mg/dl. For many people, this is not a big deal. But for others, this will be a deal breaker. For DIYers, that lack of customization will likely be frustrating. And for many families, the lack of remote data visualization may be another deal breaker. And, like with all new technology and devices, getting this stuff covered by insurance may be an uphill battle. So while optimistically this enables many people in the U.S. to finally access this technology (yay) without having to DIY, it won’t necessarily be truly available to everyone from a cost or access perspective for many years to come. So #OpenAPS and other DIY technology may still be needed from a cost/access perspective to continue to help fill gaps compared to current status quo with basic, non-connected diabetes devices (i.e. standalone pump and CGMs).
I also know that many of the parents of kids with T1D are disappointed, because the initial approval is for kids 14+, and it even notes that the system is not recommended for kids <7 or those taking less than 8u of insulin every day (usually young kids). I asked, suspecting it was related to occlusion, but it sounds more like they just don’t have enough data to say for sure that the system is safe with that small amount of insulin, and they’re working on additional studies to get data in that area.
Ditto, too, for more studies allowing different set points. They stuck with a 120mg/dl set point in order to speed to approval, but fingers crossed they get other studies done and new approvals from FDA before this device ships in the spring – that would be awesome. And I was glad to hear that they do have an “exercise” target of 150. That’s a bit of good…but I’m still hesitant that it is enough. From my personal experience knowing net IOB (here’s why net IOB matters) an hour before and when starting exercise is required information to help me decided whether or not I will need carbs to prevent lows during exercise. I don’t think this device will report on net IOB, but I admittedly haven’t seen the device and hopefully I’ll be proved wrong and the data available will be good enough for this purpose!
So in summary: this is good news. But we still need more FDA approved commercial options, and even with a single “commercial approved option”, it’s still ~6+ months away from reaching the hands of people with diabetes…so we as a #WeAreNotWaiting movement continue to have work to do to help speed up the processes for getting enhanced diabetes technology approved and available on the market, with access to view data the ways we need it.
—
*(Yes, in the title of the post I called it a commercial hybrid closed loop artificial pancreas system. It’s a hybrid closed loop, as is #OpenAPS, but it’s also on the road/part of the suite of more complex artificial pancreas technology. I realize to many PWDs “artificial pancreas” means a lot of different things. Quite certainly, regardless of definition, an artificial pancreas or hybrid closed loop still requires a lot of work. It’s not a cure by any stretch of the imagination. But it’s easy for the media to describe it as an AP, and I also find it a lot easier to describe the small device accompanying my pump when strangers ask as an “artificial pancreas” followed by an explanation rather than saying “hybrid closed loop”.
If anything, I think having the media broadly categorize it as an AP will encourage the diabetes community to ask more questions about what exactly this tech does, leading to greater understanding and better expectations about what the device will/won’t be able to do. So this may result in a good thing.)