There’s a secret behind why #OpenAPS was able to deal so well with my BGs during norovirus. Namely, “autosensitivity”.
Autosensitivity (or “autosens”, for short hand) is an advanced feature that can optionally be enabled in OpenAPS.
We know how hard it is for a PWD (person with diabetes) to pay attention to all the numbers and all the things and realize when something is “off”. This could be a bad pump site, a pump site going bad, hormones from growth, hormones from menstrual cycles, sensitivity from exercise the day before, etc. So at the beginning of the year, Scott and I started brainstorming with the community about automatically detecting when the PWD is more or less sensitive to insulin than normal, and adjusting accordingly. Building on the success we’d had in DIYPS with fixed “sensitivity” and “resistance” modes, we built the feature to assess how sensitive or resistant the body is (compared to normal), rather than just a binary mode that sets a predefined response.
How OpenAPS calculates autosensitivity/how it works
It looks at each BG data point for the last 24 hours and calculates the delta (actual observed change) over the last 5 minutes. It then compares it to “BGI” (blood glucose impact, which is how much BG *should* be dropping from insulin alone), and assesses the “deviations” (differences between the delta and BGI).
When sensitivity is normal and basals are well tuned, we expect somewhere between 45-50% of non-meal deviations to be negative, and the remaining 50-55% of deviations should be positive. (To exclude meal-related deviations, we exclude overly large deviations from the sample.) So if you’re outside of that range, you are probably running sensitive or resistant, and we want to adjust accordingly. The output of the detect-sensitivity code is a single ratio number, which is then used to adjust both the baseline basal rate as well as the insulin sensitivity factor (and, optionally, BG targets).
Autosens is designed to detect to food-free downward drift, due to basal rates being too high for the current state of the body, and will adjust basals downward to compensate. The other meal-assist related portion of the algorithms do a pretty good job of dealing with larger than expected post-meal spikes due to resistance: auto-sensitivity mostly comes into play for resistance when you’re sick or otherwise riding high even without food.
Does this calculate basals?
No. Similar to everything else in OpenAPS, this works from your established basals – meaning the baseline basal rates in your pump are what the sensitivity calculations are adjusting from. If you run a marathon and your sensitivity is normally 40, it might adjust your sensitivity to 60 (meaning 1u of insulin would drop your BG an expected 60mg/dl instead of 40 mg/dl) and temporarily adjust your baseline basal rate of 1u to .6u/hour, for example.
This algorithm is simply saying “there’s something going on, let’s adjust proportionately to deal with the lower-than-usual or higher-than-usual sensitivity, regardless of cause”. It easily detects “your basals are too high and/or your ISF is too low” or “your basals are too low and/or your ISF is too high”, but actually differentiating between the effect of basal and ISF is a bit more difficult to do with a simple algorithm like this, so we’re working on a number of new algorithms and tools (see “oref0 issue 99” for our brainstorming on basal tuning and the subsequent issues linked from there) to tackle this in the future.
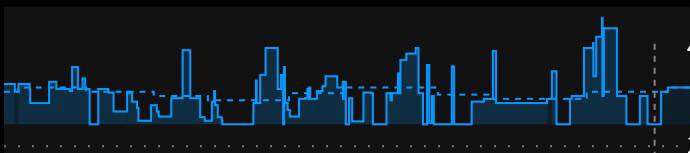
#OpenAPS’s autosensitivity adjustments during norovirus
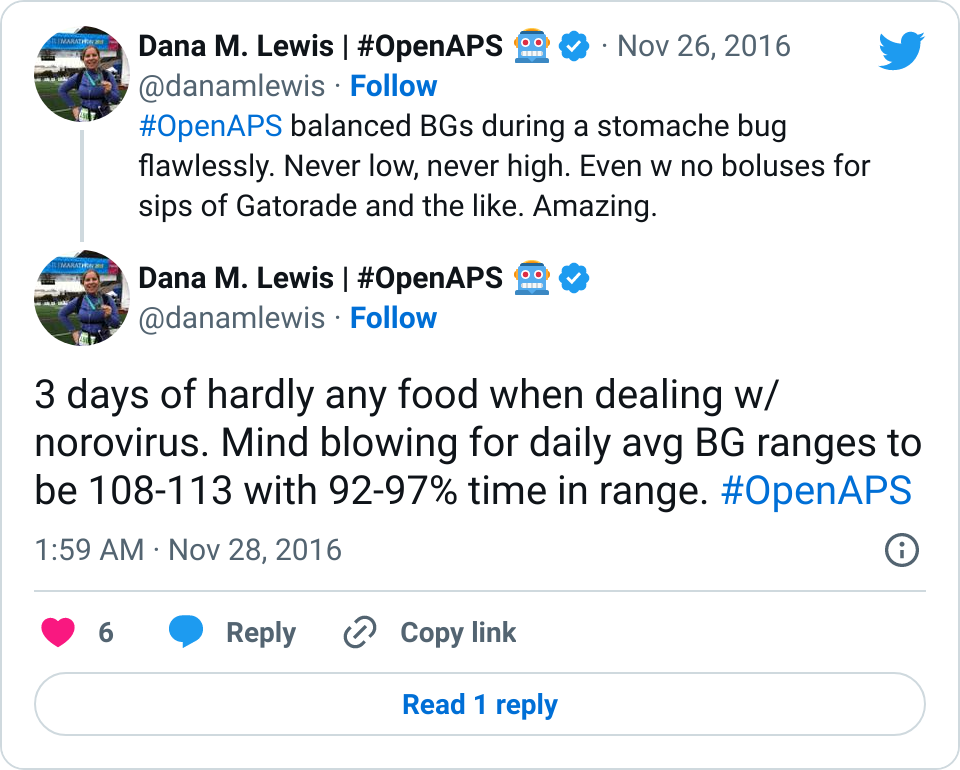
After I got over the worst of the norovirus, I started looking at what OpenAPS was calculating for my sensitivity during this time. I was especially curious what would happen during the 2-3 days when I was eating very little.
My normal ISF is 40, but OpenAPS gradually calculated the shift in my sensitivity all the way to 50. That’s really sensitive, and in fact I don’t remember ever seeing a sensitivity adjustment that dramatic – but makes sense given that I usually don’t go so long without eating. (Usually when I notice I’m a little sensitive, I’ll check and see that autosens has been adjusting based on an estimated 43 or so sensitivity.)
And in later days, as expected when sick, I shifted to being more resistant. So autosens continued to assess the data and began adjusting to an estimated sensitivity of 38 as my body continued fighting the virus.
—
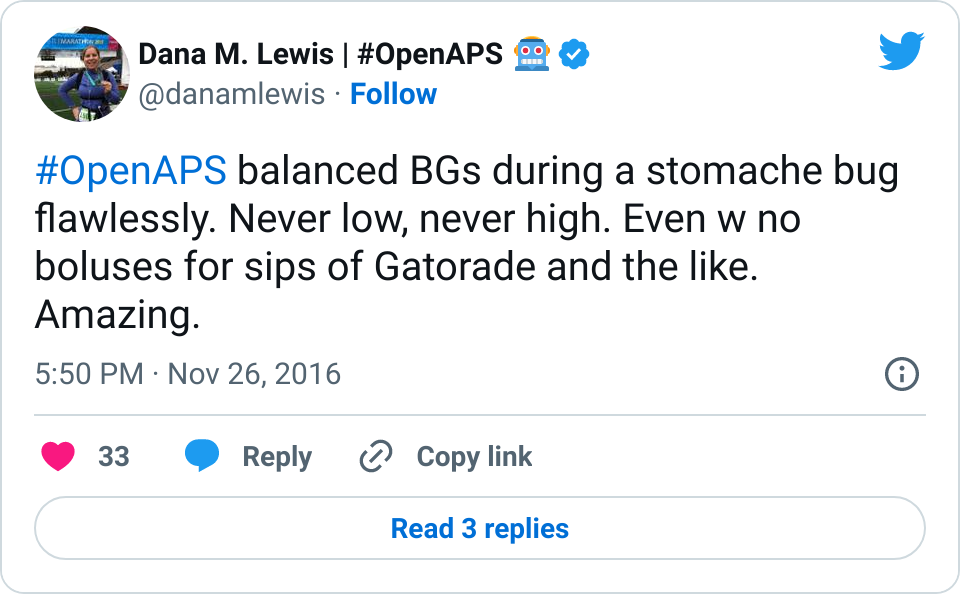
It is so nice to have the tools to automatically make these assessments and adjustments, rather than having to manually deal with them on top of being sick!



Recent Comments