If you’re feeling overwhelmed by the rapid development of AI, you’re not alone. It’s moving fast, and for many people the uncertainty of the future (for any number of reasons) can feel scary. One reaction is to ignore it, dismiss it, or assume you don’t need it. Some people try it once, usually on something they’re already good at, and when AI doesn’t perform better than they do, they conclude it’s useless or overhyped, and possibly feel justified in going back to ignoring or rejecting it.
But that approach misses the point.
AI isn’t about replacing what you already do well. It’s about augmenting what you struggle with, unlocking new possibilities, and challenging yourself to think differently, all in the pursuit of enabling YOU to do more than you could yesterday.
One of the ways to navigate the uncertainty around AI is to shift your mindset. Instead of thinking, “That’s hard, and I can’t do that,” ask yourself, “What if I could do that? How could I do that?”
Sometimes I get a head start by asking an LLM just that: “How would I do X? Layout a plan or outline an approach to doing X.” I don’t always immediately jump to doing that thing, but I think about it, and probably 2 out of 3 times, laying out a possible approach means I do come back to that project or task and attempt it at a later time.
Even if you ultimately decide not to pursue something because of time constraints or competing priorities, at least you’ve explored it and possibly learned something even in the initial exploration about it. But, I want to point out that there’s a big difference between legitimately not being able to do something and choosing not to. Increasingly, the latter is what happens, where you may choose not to tackle a task or take on a project: this is very different from not being able to do so.
Finding the Right Use Cases for AI
Instead of testing AI on things you’re already an expert in, try applying it to areas where you’re blocked, stuck, overwhelmed, or burdened by the task. Think about a skill you’ve always wanted to learn but assumed was out of reach. Maybe you’ve never coded before, but you’re curious about writing a small script to automate a task. Maybe you’ve wanted to design a 3D-printed tool to solve a real-world problem but didn’t know where to start. AI can be a guide, an assistant, and sometimes even a collaborator in making these things possible.
For example, I once thought data science was beyond my skill set. For the longest time, I couldn’t even get Jupyter Notebooks to run! Even with expert help, I was clearly doing something silly and wrong, but it took a long time and finally LLM assistance to get step by step and deeper into sub-steps to figure out the step that was never in the documentation or instructions that I was missing – and I finally figured it out! From there, I learned enough to do a lot of the data science work on my own projects. You can see that represented in several recent projects. The same thing happened with iOS development, which I initially felt imposter syndrome about. And this year, after FOUR failed attempts (even 3 using LLMs), I finally got a working app for Android!
Each time, the challenge felt enormous. But by shifting from “I can’t” to “What if I could?” I found ways to break through. And each time AI became a more capable assistant, I revisited previous roadblocks and made even more progress, even when it was a project (like an Android version of PERT Pilot) I had previously failed at, and in that case, multiple times.
Revisiting Past Challenges
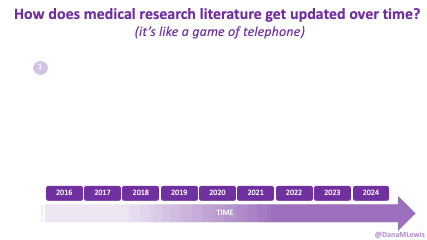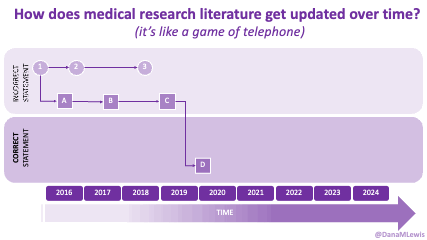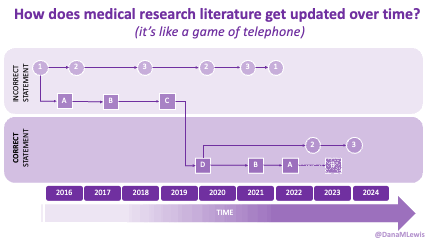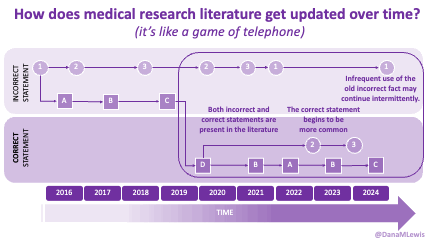
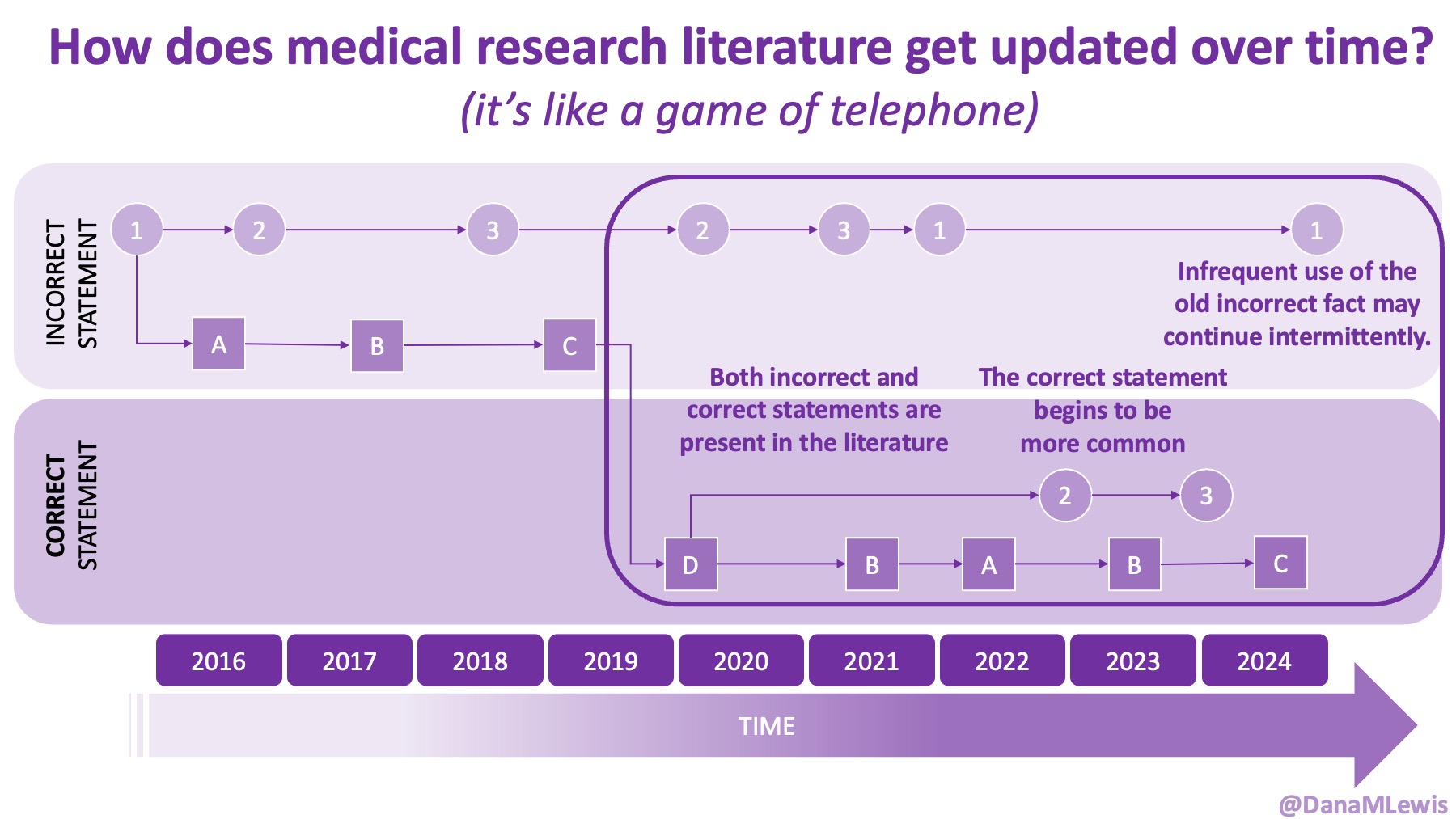
AI is evolving rapidly, and what wasn’t possible yesterday might be feasible today. Literally. (A great example is that I wrote a blog post about how medical literature seems like a game of telephone and was opining on AI-assisted tools to aid with tracking changes to the literature over time. The day I put that blog post in the queue, OpenAI announced their Deep Research tool, which I think can in part address some of the challenges I talked about currently being unsolved!)
One thing I have started to do that I recommend is keeping track of problems or projects that feel out of reach. Write them down. Revisit them every few months, and explore them with the latest LLM and AI tools. You might be surprised at how much has changed, and what is now possible.
Moving Forward with AI
You don’t even have to use AI for everything. (I don’t.) But if you’re not yet in the habit of using AI for certain types of tasks, I challenge you to find a way to use an LLM for *something* that you are working on.
A good place to insert this into your work/projects is to start noting when you find yourself saying or thinking “this is the way we/I do/did things”.
When you catch yourself thinking this, stop and ask:
- Does it have to be done that way? Why do we think so?
- What are we trying to achieve with this task/project?
- Are there other ways we can achieve this?
- If not, can we automate some or more steps of this process? Can some steps be eliminated?
You can ask yourself these questions, but you can also ask these questions to an LLM. And play around with what and how you ask (the prompt, or what you ask it, makes a difference).
One example for me has been working on a systematic review and meta analysis of a medical topic. I need to extract details about criteria I am analyzing across hundreds of papers. Oooph, big task, very slow. The LLM tools aren’t yet good about extracting non-obvious data from research papers, especially PDFs where the data I am interested may be tucked into tables, figure captions, or images themselves rather than explicitly stated as part of the results section. So for now, that still has to be manually done, but it’s on my list to revisit periodically with new LLMs.
However, I recognized that the way I was writing down (well, typing into a spreadsheet) the extracted data was burdensome and slow, and I wondered if I could make a quick simple HTML page to guide me through the extraction, with an output of the data in CSV that I could open in spreadsheet form when I’m ready to analyze. The goal is easier input of the data with the same output format (CSV for a spreadsheet). And so I used an LLM to help me quickly build that HTML page, set up a local server, and run it so I can use it for data extraction. This is one of those projects where I felt intimidated – I never quite understood spinning up servers and in fact didn’t quite understand fundamentally that for free I can “run” “a server” locally on my computer in order to do what I wanted to do. So in the process of working on a task I really understood (make an HTML page to capture data input), I was able to learn about spinning up and using local servers! Success, in terms of completing the task and learning something I can take forward into future projects.
Another smaller recent example is when I wanted to put together a simple case report for my doctor, summarizing symptoms etc, and then also adding in PDF pages of studies I was referencing so she had access to them. I knew from the past that I could copy and paste the thumbnails from Preview into the PDF, but it got challenging to be pasting 15+ pages in as thumbnails and they were inserting and breaking up previous sections, so the order of the pages was wrong and hard to fix. I decided to ask my LLM of choice if it was possible to automate compiling 4 PDF documents via a command line script, and it said yes. It told me what library to install (and I checked this is an existing tool and not a made up or malicious one first), and what command to run. I ran it, it appended the PDFs together into one file the way I wanted, and it didn’t require the tedious hand commands to copy and paste everything together and rearrange when the order was messed up.
The more I practice, the easier I find myself switching into the habit of saying “would it be possible to do X” or “Is there a way to do Y more simply/more efficiently/automate it?”. That then leads to portions which I can decide to implement, or not. But it feels a lot better to have those on hand, even if I choose not to take a project on, rather than to feel overwhelmed and out of control and uncertain about what AI can do (or not).
 If you can shift your mindset from fear and avoidance to curiosity and experimentation, you might discover new skills, solve problems you once thought were impossible, and open up entirely new opportunities.
If you can shift your mindset from fear and avoidance to curiosity and experimentation, you might discover new skills, solve problems you once thought were impossible, and open up entirely new opportunities.
So, the next time you think, “That’s too hard, I can’t do that,” stop and ask:
“What if I could?”
—
If you appreciated this post, you might like some of my other posts about AI if you haven’t read them.
 TLDR: Instead of asking “Which model is best?”, a better question might be:
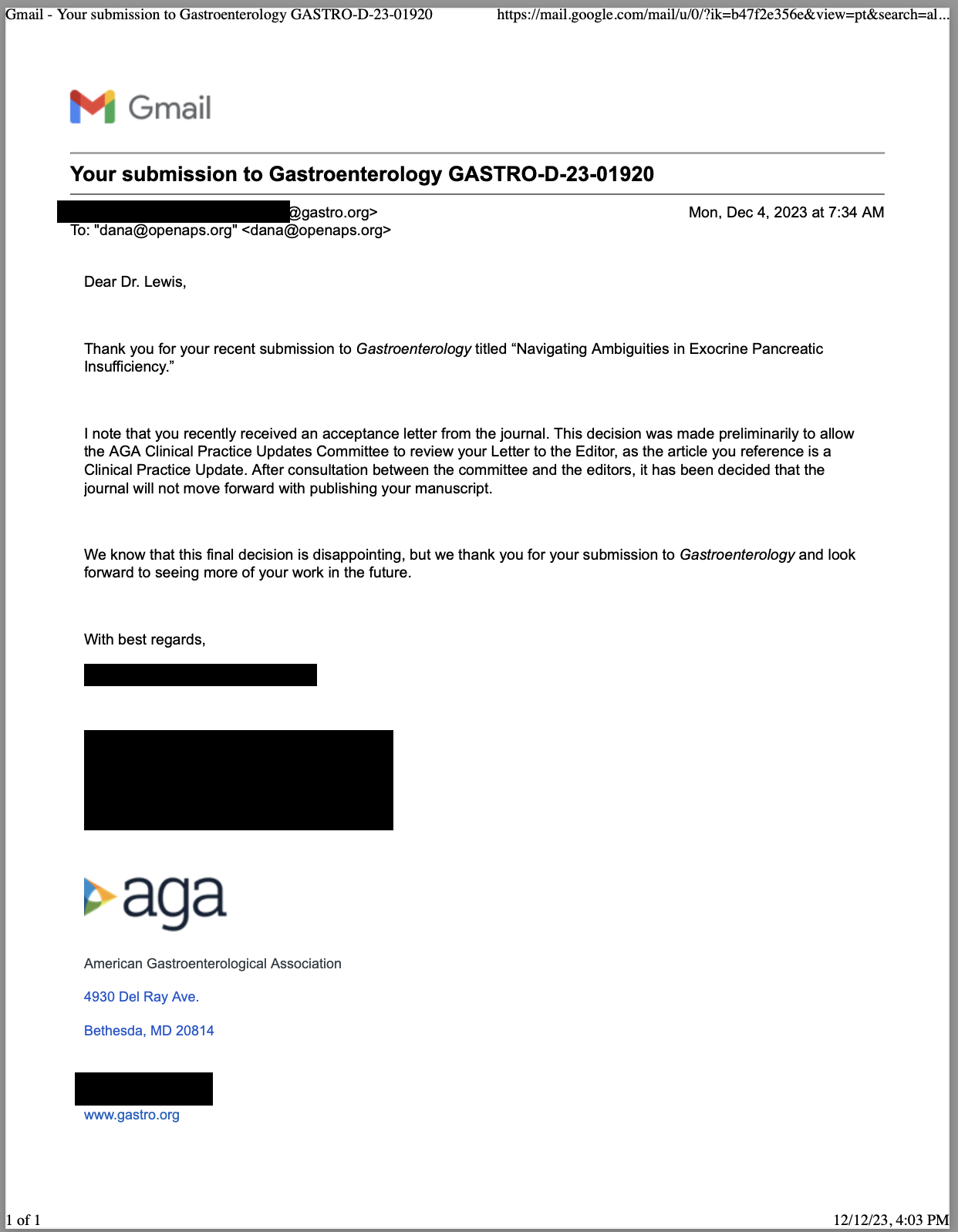
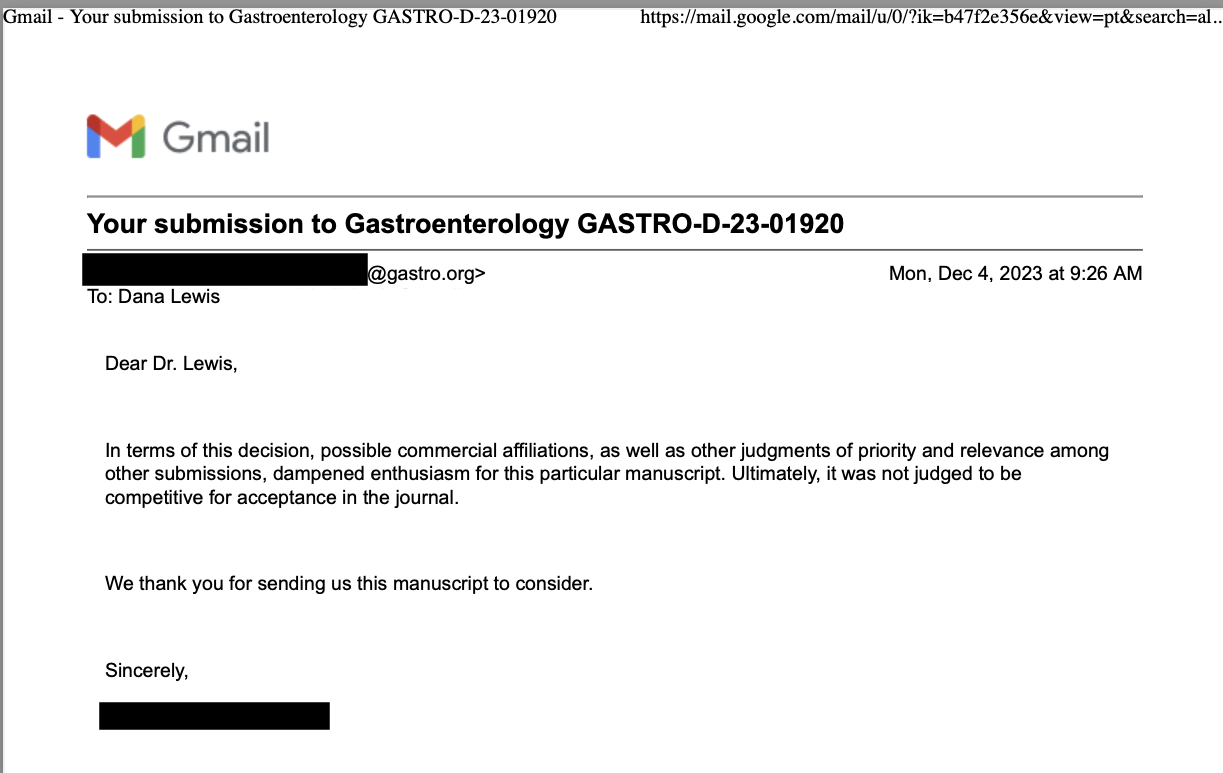
TLDR: Instead of asking “Which model is best?”, a better question might be: As an example for how I like to disseminate my articles personally, every time a journal article is published and I have access to it, I updated
As an example for how I like to disseminate my articles personally, every time a journal article is published and I have access to it, I updated 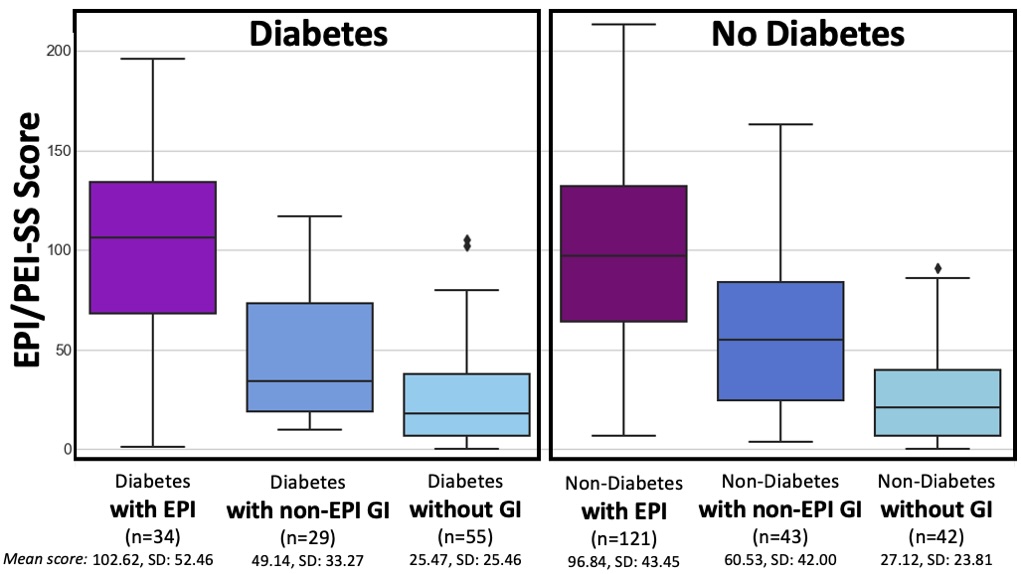
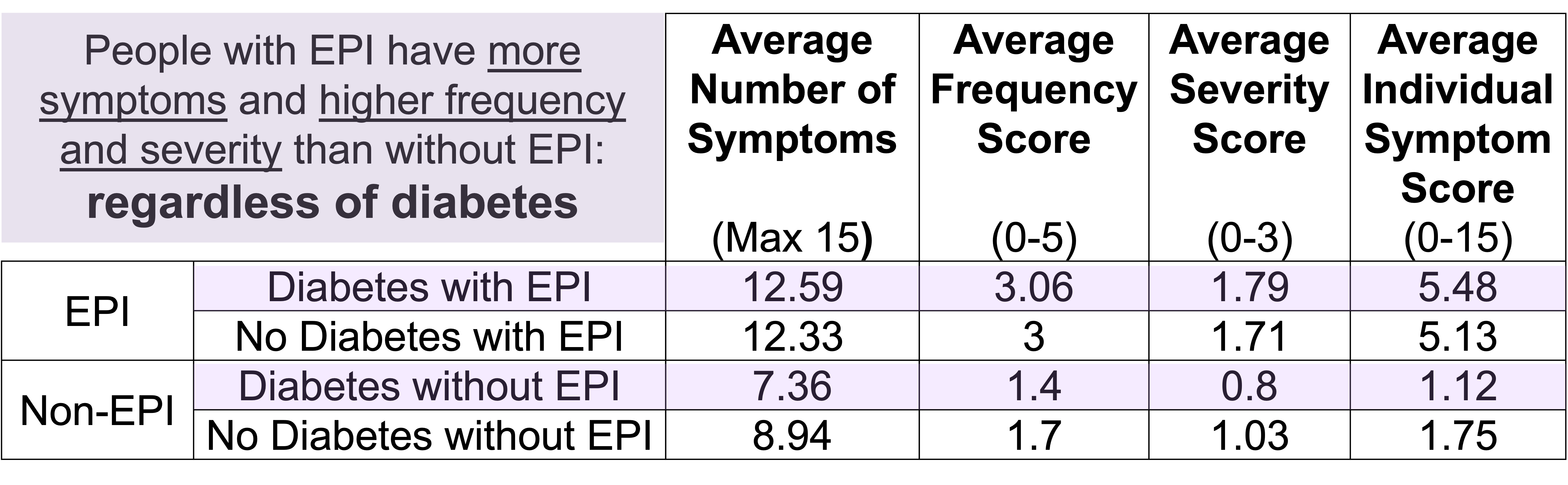
 How do you have people take the EPI/PEI-SS? You can pull this link up (
How do you have people take the EPI/PEI-SS? You can pull this link up (





Recent Comments