One of the things I wish people would consider more often when thinking about AI is how they can use it to scale themselves. What are some time-consuming things that they currently have to do themselves that AI could do for them to streamline their output and increase their productivity? Productivity for giving them more time to do the things only they can do, the things they want to do, or the things they love to do. (And to help stop procrastinating on things they have to do.)
I have a habit of trying to scale myself. These days, it’s often related to EPI (exocrine pancreatic insufficiency, which some areas of the world know by the acronym PEI). I developed a strong knowledge base first from personal experience, then by doing research – including a systematic review where I read hundreds, plural, of research papers on key topics related to design protocols and guidelines. As a result of both personal and research experience, I have a lot of knowledge. It gets tapped almost daily in the EPI support groups that I’m a part of.
Whenever I notice myself answering the same question repeatedly, I make a mental note of it. Eventually, if a topic comes up often enough, I turn my response into a blog post. This way, I can provide a well-structured, comprehensive answer with more time and context than a quick comment on social media allows – and with the ability to give the same, high quality answer to multiple people (and in some cases, hundreds or thousands of people rather than the few who might see the comment buried in a response thread).
A few examples of this include:
- Explaining why fecal elastase test results change over time; why they’re not perfect; what being above or below certain cutoffs mean; why you don’t need to stop taking enzymes for an elastase test; why elastase test isn’t directly impacted by taking enzymes; why elastase isn’t used to determine how much enzymes you need or whether or not enzymes are ‘working for you’. All that in one post (here) with a lot of context and resources including links to medical literature to back all that up, which is hard to do well as one-off facebook comments and easier to do in a blog post format.
- A guide to discussing enzyme dosing starting guidelines and best practices for titrating (increasing) your enzyme dose, including resources (again, medical literature) to share with your doctor to help inform your discussions
- Information on an approach to record what you’re eating, and why, in order to adjust and optimize your enzyme dosing, because enzymes have to (should) be dosed in relationship to the food consumed.
One of my favorite things with this approach is then seeing other people begin to share the links to my longer-form content to help answer common questions. By writing things down in a shareable way, it also enables and supports other people to scale your work by sharing it easily. This has started to happen more and more with the elastase blog post, in part because there are so few resources that cover this information all in one place.
For me, I lean toward writing, but for other people that could be videos, podcast/audio recording, or other formats that can capture things you know and make them shareable, thus scaling yourself.
For me, this approach of “scaling myself” and thinking about longer form content to post online instead of re-typing similar answers over and over again isn’t unique to EPI.
I have been doing this for over a decade. I developed this pattern early after we developed and shared OpenAPS (the first open source automated insulin delivery algorithm) with the world. Early on, I found myself answering the same technical questions repeatedly in online discussions with the same answers. Typing out explanations on my phone was inefficient, and if one person had a question, others likely had the same one. Instead of repeating myself, I took the time to document answers. I would often pause, write up the information in the documentation, and share that instead. This made it easier and quicker to go find and share a link instead of retyping responses, and it also took less time, so I was willing to do it more quickly than if I had to delay what I was doing in real life in order to type out a long yet already-answered question. Over time, I had to do less one-off typing on my phone (and could save that time and energy for true, one-off unique questions) and could share links with a lot more information more easily.
How do I use AI to scale this type of work?
A lot of the above tasks are related to writing. There are different ways you can use AI for writing, without having it write something completely. You can give it notes – whether you type or voice dictate them – and have it clean up your notes, so you can focus on thinking and not about typing or fixing typos that break your flow. You can have it convert the notes into full sentences. You can ask it to write a paragraph or an article based on the notes. You can ask it to suggest wording for a particular sentence you want to clarify for your audience.
If you think about the AI as an intern and/or a partner/collaborator who you would ask to review or edit for you, you’ll likely find even more ways to integrate AI into different parts of your writing process, even if it’s not doing the full writing for you.
I have also tried to task the AI with writing for me, with mixed results. This doesn’t mean I don’t use it, but I’ve been practicing and learning where it generates usable content and where it doesn’t.
A lot of it depends on the prompt and the topic (as much as it does the output in terms of style, length, intended audience etc).
If it’s a topic that’s “known”, it can write more content that I can take and edit and transform, as opposed to when I am trying to write about a concept that is far from the current knowledge base. (I mean far for both humans and of AI – a lot of my work is bleeding edge, pushing fields towards new developments and leading humans there.) Sometimes I ask it to write something and end up using none of the content, but by saying “ugh no” my brain has jumped to saying to myself “it should really say…” and I am able to more quickly springboard into manually writing the content I was previously slow on. In other words, it can be a brainstorming tool in the opposite sense, showing me what I do not want to say on a topic! And on some of my frontier/bleeding edge topics, it reflects what is commonly ‘known’ and when what is known is now wrong (example, as always, of how it’s commonly incorrectly reported that chronic pancreatitis is the most common cause of EPI), it helps me more clearly distinguish the new content from the old, wrong, or misinformed.
(Also, it’s worth reminding you what I have to remind myself, that AI is changing constantly and new tools override what is known about what tasks do and don’t do well! For example, in between writing this and posting it, OpenAI released GPT4.5, which is reportedly better at writing-related tasks than GPT-4o and other older models. I’ll have to test it and see if that’s true and for what kinds of writing tasks!)
This isn’t the only way you can scale yourself with AI, though. Scaling yourself doesn’t have to be limited to writing and documentation style tasks. AI and other tools can help with many tasks (more examples here and here), such as:
- Cleaning and transforming data into different formats
- Converting a CSV file into a more readable table
- Writing code to automate tedious data processing
- Drafting plain-language instructions for engineers or programmers
- Checking whether instructions or explanations are clear and understandable, and identifying any gaps in logic that you missed on your first pass
By leveraging AI and other automation tools, you can free up time and energy for higher-value work: the things you are uniquely suited to do in the world, and the things that you want or love to do. And do them more easily!
Pro tip: if you find yourself procrastinating a task, this may be a good sign that you could use AI for some of it.
I’m trying to use noticing procrastination as a trigger for considering AI for a task.
An example of this is an upcoming post with a bunch of math and meaty cost analysis that I originally did by hand. I needed (wanted) to re-do these estimates with different numbers, but procrastinated a bit because having to carefully re-do all the estimates and replace them throughout the blog post seemed tedious, so my brain wanted to procrastinate. So, I took the blog post and dumped it in with a prompt asking it to write Jupyter Notebook code to replicate the analyses explained via the plain language post, with the ability to adjust all input variables and see the results in a table so I could compare the original and updated numbers. It took less than 1 minute to generate this code and about 5 minutes for me to copy/paste, update the numbers, run it, and evaluate the output and decide what to update in the post. Manually, this would’ve taken 30-60 minutes due to needing to check my work manually and trace it throughout the post. Instead, this automated the tedious bit and will result in this new post coming out next week rather than weeks from now (read about it here – it’s an analysis on how cost-effect Life for a Child is, a charity supporting people living with diabetes in low- and middle-income countries that can use your help to save lives.)
 I encourage you to think about scaling yourself and identifying a task or series of tasks where you can get in the habit of leveraging these tools to do so. Like most things, the first time or two might take a little more time. But once you figure out what tasks or projects are suited for this, the time savings escalate. Just like learning how to use any new software, tool, or approach. A little bit of invested time up front will likely save you a lot of time in the future.
I encourage you to think about scaling yourself and identifying a task or series of tasks where you can get in the habit of leveraging these tools to do so. Like most things, the first time or two might take a little more time. But once you figure out what tasks or projects are suited for this, the time savings escalate. Just like learning how to use any new software, tool, or approach. A little bit of invested time up front will likely save you a lot of time in the future.
 If you have been prescribed pancreatic enzyme replacement therapy (PERT), aka enzymes for exocrine pancreatic insufficiency (EPI or PEI), you may be wondering how long it will take before you start to feel better or it starts to work. This is a common question, and the answer depends on several factors, including the dosage, meal composition, and how well your body uses the enzymes. Some improvements can be seen within a single meal, while other benefits take longer to manifest. It also depends on whether you have EPI, or if you have EPI in concert with other types of gastrointestinal conditions, because some of your symptoms may be coming from other conditions.
If you have been prescribed pancreatic enzyme replacement therapy (PERT), aka enzymes for exocrine pancreatic insufficiency (EPI or PEI), you may be wondering how long it will take before you start to feel better or it starts to work. This is a common question, and the answer depends on several factors, including the dosage, meal composition, and how well your body uses the enzymes. Some improvements can be seen within a single meal, while other benefits take longer to manifest. It also depends on whether you have EPI, or if you have EPI in concert with other types of gastrointestinal conditions, because some of your symptoms may be coming from other conditions.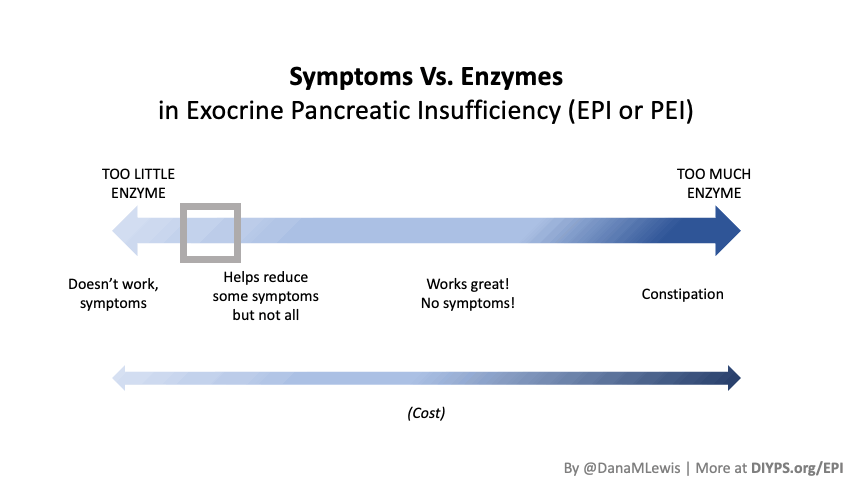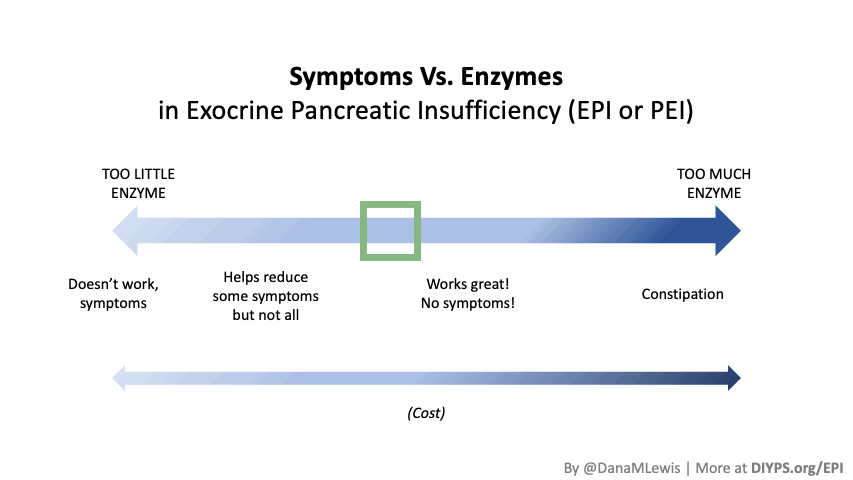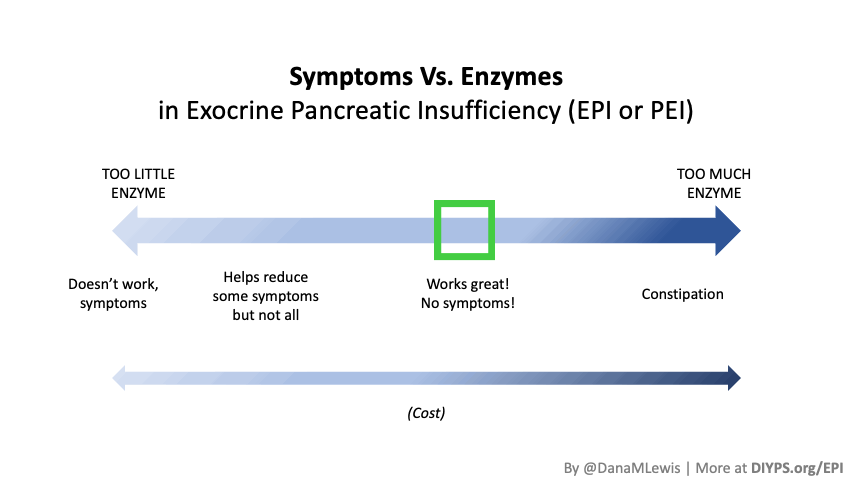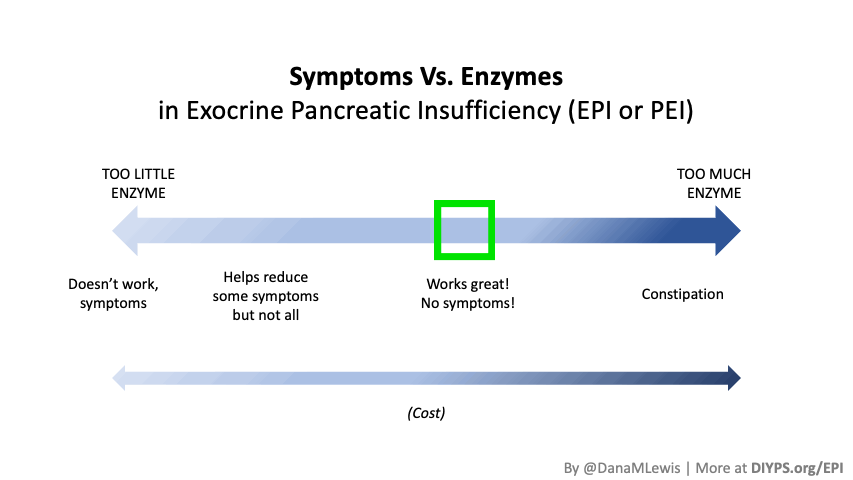
 TL;DR: Instead of arbitrarily lowering or increasing fat or fiber in the diet, measure and estimate what you are consuming first. If you have EPI,
TL;DR: Instead of arbitrarily lowering or increasing fat or fiber in the diet, measure and estimate what you are consuming first. If you have EPI,  It occurred to me that maybe I could tweak it somehow and make the bullets of the list represent food items. I wasn’t sure how, so I asked the LLM if it was possible. Because I’ve done my other ‘design’ work in PowerPoint, I went there and quickly dropped some shapes and lines to simulate the icon, then tested exporting – yes, you can export as SVG! I spent a few more minutes tweaking versions of it and exporting it. It turns out, yes, you can export as SVG, but then the way I designed it wasn’t really suited for SVG use. When I had dropped the SVG into XCode, it didn’t show up. I asked the LLM again and it suggested trying PNG format. I exported the icon from powerpoint as PNG, dropped it into XCode, and it worked!
It occurred to me that maybe I could tweak it somehow and make the bullets of the list represent food items. I wasn’t sure how, so I asked the LLM if it was possible. Because I’ve done my other ‘design’ work in PowerPoint, I went there and quickly dropped some shapes and lines to simulate the icon, then tested exporting – yes, you can export as SVG! I spent a few more minutes tweaking versions of it and exporting it. It turns out, yes, you can export as SVG, but then the way I designed it wasn’t really suited for SVG use. When I had dropped the SVG into XCode, it didn’t show up. I asked the LLM again and it suggested trying PNG format. I exported the icon from powerpoint as PNG, dropped it into XCode, and it worked! If you can shift your mindset from fear and avoidance to curiosity and experimentation, you might discover new skills, solve problems you once thought were impossible, and open up entirely new opportunities.
If you can shift your mindset from fear and avoidance to curiosity and experimentation, you might discover new skills, solve problems you once thought were impossible, and open up entirely new opportunities.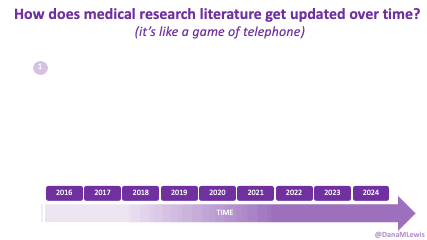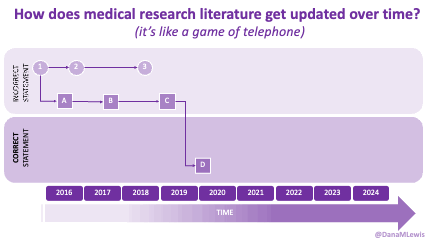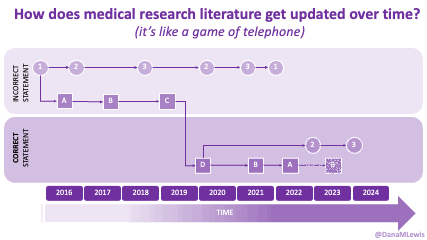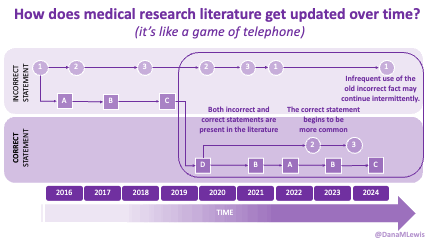
 TLDR: Instead of asking “Which model is best?”, a better question might be:
TLDR: Instead of asking “Which model is best?”, a better question might be: AI can be useful for reducing clinician workload and improving documentation efficiency. But like any tool, its impact depends on how it’s implemented, how transparent the process is, and whether there are safeguards to address errors when they occur.
AI can be useful for reducing clinician workload and improving documentation efficiency. But like any tool, its impact depends on how it’s implemented, how transparent the process is, and whether there are safeguards to address errors when they occur. This basically has been our plan for if either of us were to get COVID-19 (or the flu), and it’s good to know this plan works for a variety of conditions including RSV and the common cold. The main thing we would do differently in the future is that Scott should have masked the very first night he had symptoms of RSV, and he has decided that he’ll be masking any time he’s in the same room as someone who’s been coughing, as that’s considerably less annoying than being sick. (He really did not like the experience of having RSV.) I obviously did not get it from that first night when he first had the most minor symptoms of RSV, but that was probably the period of highest risk of transmission of either week, given the subsequent precautions we took after that.
This basically has been our plan for if either of us were to get COVID-19 (or the flu), and it’s good to know this plan works for a variety of conditions including RSV and the common cold. The main thing we would do differently in the future is that Scott should have masked the very first night he had symptoms of RSV, and he has decided that he’ll be masking any time he’s in the same room as someone who’s been coughing, as that’s considerably less annoying than being sick. (He really did not like the experience of having RSV.) I obviously did not get it from that first night when he first had the most minor symptoms of RSV, but that was probably the period of highest risk of transmission of either week, given the subsequent precautions we took after that. It sounds nice. Hopeful. Productive. It implies the lemons are gently handed to you. They’re probably clean, ripe, and maybe sitting in a cute little basket. Like life’s just giving you a quirky unexpected opportunity.
It sounds nice. Hopeful. Productive. It implies the lemons are gently handed to you. They’re probably clean, ripe, and maybe sitting in a cute little basket. Like life’s just giving you a quirky unexpected opportunity. And sometimes people do. That’s valid. Sometimes I’ve done that.
And sometimes people do. That’s valid. Sometimes I’ve done that. Forget the default script. There’s no “right” way to respond.
Forget the default script. There’s no “right” way to respond.
Recent Comments