I’ve had the opportunity to meet some fantastic people through our work with #DIYPS and #OpenAPS, one of whom is Eric Von Hippel, an MIT professor and researcher who work on user innovation. He shared a great example of user innovation that I was previously unfamiliar with, but is an awesome parallel for what we in the diabetes community are doing.
History: bike manufacturers used to make bikes for riding on flat surfaces. Some people wanted to ride their bikes down mountains, but existing bikes weren’t too comfortable (they didn’t have spring-based seats – ouch!). So, bikers started customizing and modifying the bikes they had. Eventually, bike manufacturers saw the demand and started building mountain bikes with the same features that the original mountain bikers had used. (And if you don’t like my paraphrased version of this story, Wikipedia is always your friend!)
The parallels:
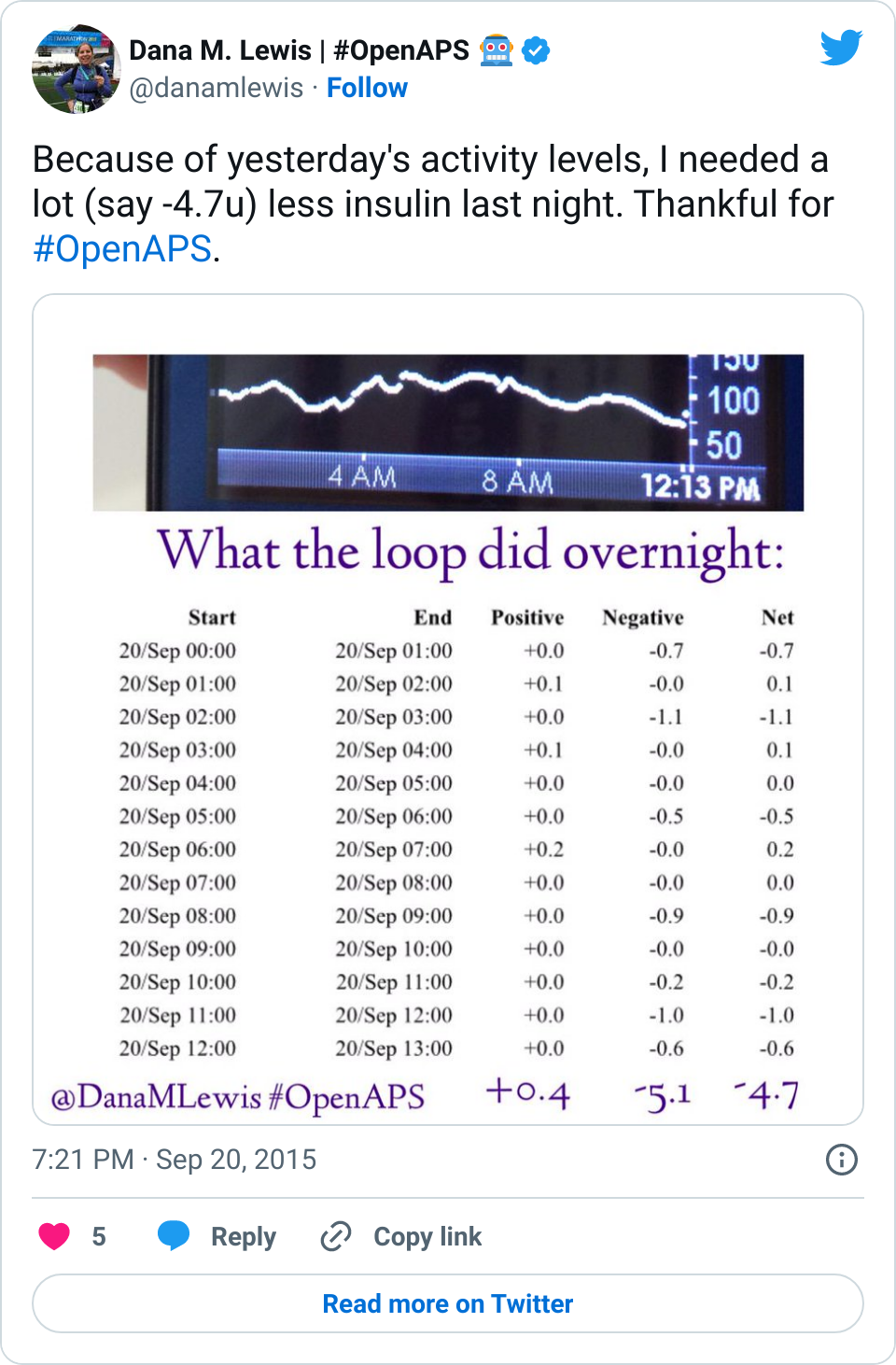
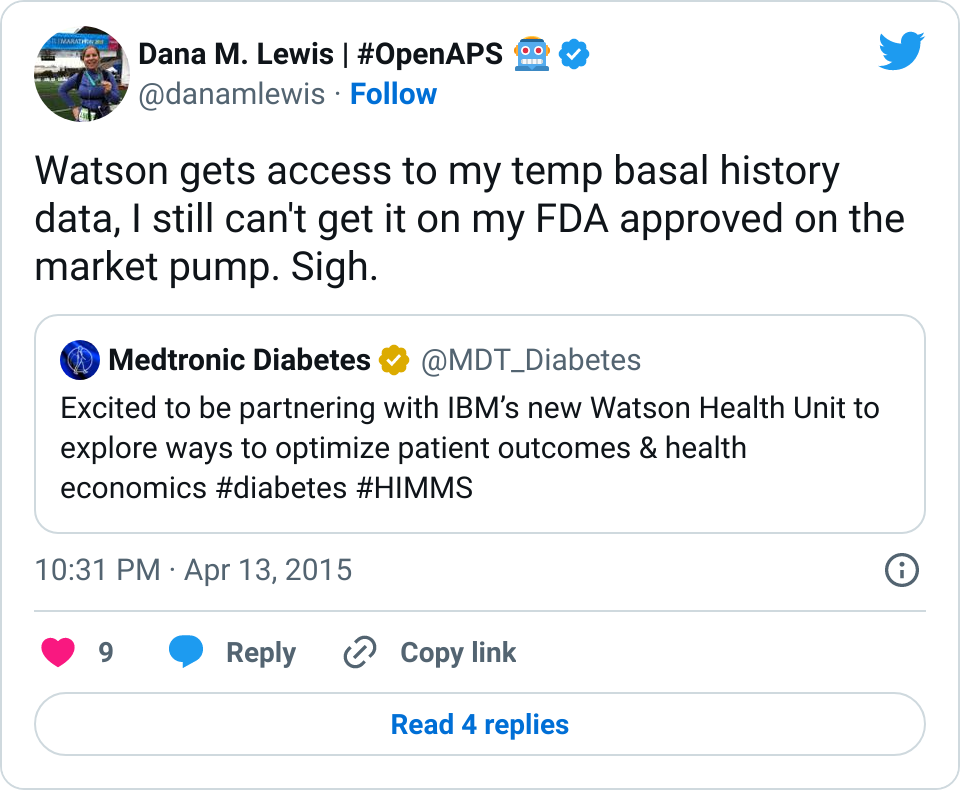
We in the diabetes community have seen a series of needs that are not being met with our existing, FDA approved medical devices that are out on the market. From not-loud-enough alarms to not enabling us to track critical information like temporary basal rate history on the pump itself, these are the needs that drove me (and Scott) to first build #DIYPS and then to close the loop. At the same time, the need for remote data access is what drove John Costik and then the other Nightscout founders and developers to build an amazing, community-based open source tool to enable real-time remote data sharing.
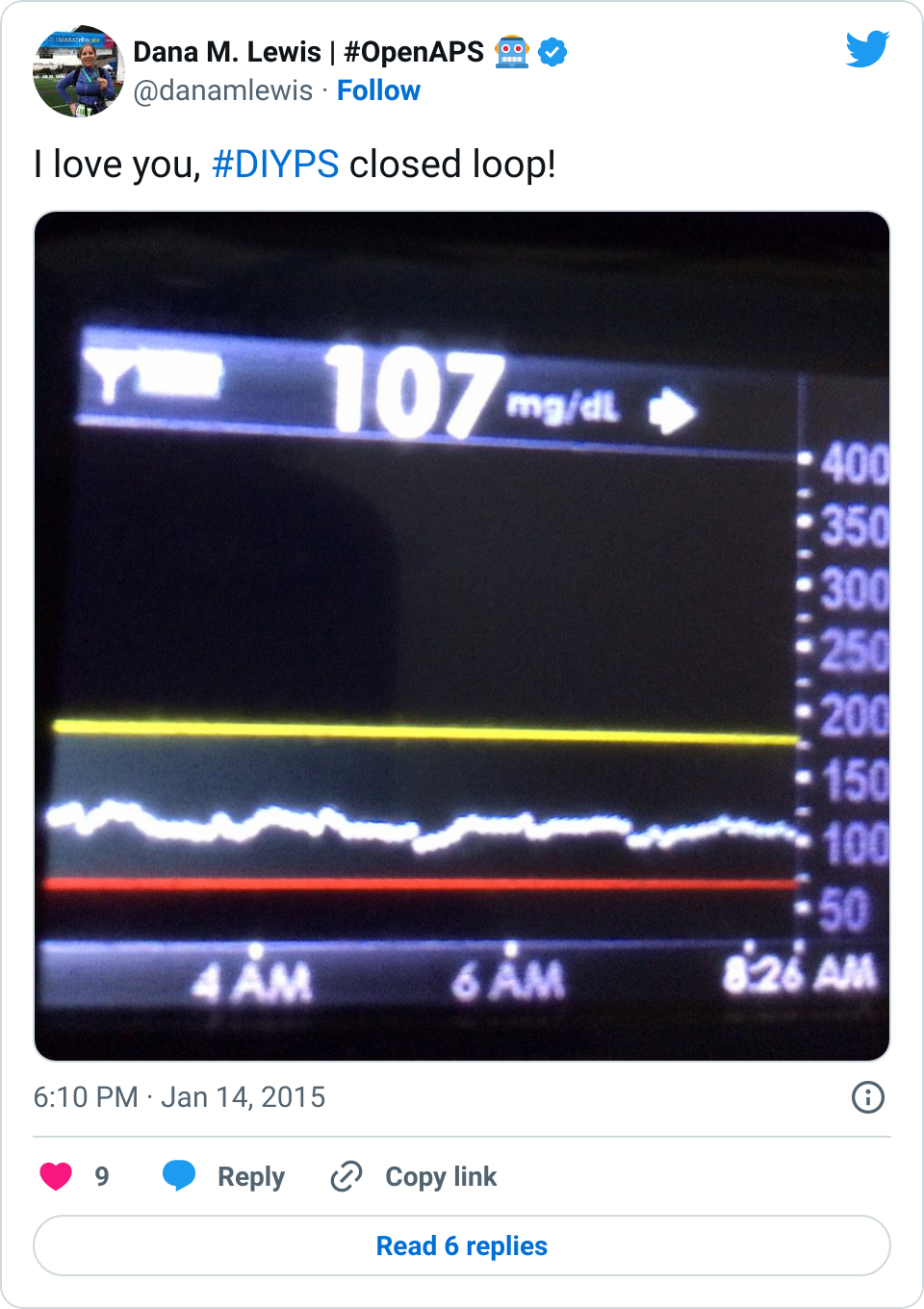
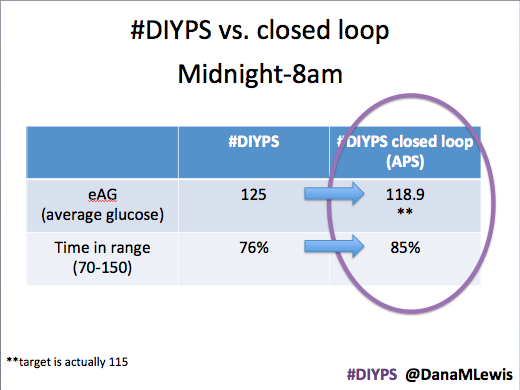
Are there commercial products coming to market or are in the market that meet some of these needs now? Sure. But remember, I’ve had diabetes since 2002. In 2013, when Scott and I first started working to solve my need for louder alarms, there was NO commercial solution available for either remote data access or alarm customization. Thus the need for #DIYPS, which we built in 2013, and Nightscout, which blossomed in early 2014. And even though tools like Dexcom SHARE and MiniMed Connect have come to market (and in some cases, more quickly with help from the community communicating to the FDA about the critical importance of these tools), they came in 2015, which is a long time to wait for new tools when you’re dealing with diabetes 24/7/365. And when we managed to close the loop, again with help from the amazing #wearenotwaiting community, in December of 2014? Well, it’s now nearing the end of a year (and with amazing continued results from #OpenAPS not just for me, but for 13 additional people and potentially more to come soon), and we are AT LEAST a year and a half, if not more, away from any commercial device to reach the market. Not to mention: I’m not sure that the first generation of closed loops commercially available will be good enough for me.
The commercial entities are getting there. And, I always want to give them credit – I have a closed loop, but I can’t have one without a solidly working insulin pump and an excellent CGM system. They are, for the most part (with the exception of some missing features), making good, solid safe products for me to use.
The manufacturers are also starting to be open to more conversations. Not just “listening”, which they’ve sort-of/maybe done in drips over the past, but actual two-way conversations where we can share the needs that we know of in the community, and discuss what can be incorporated into their commercial product pipeline more quickly. This is progress starting to be made, and I’m excited to see more of it. It seems like there is a refreshed mindset and energy in the industry, as well as an understanding that we all care deeply about safety and that we’re all in this together to make life with diabetes less of a burden – like riding downhill on a mountain bike rather than a road bike.

Recent Comments